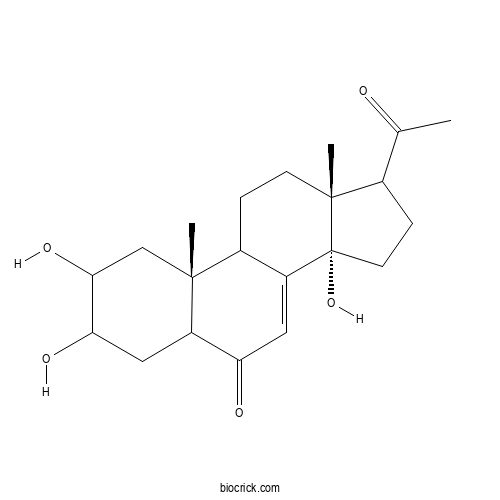
PoststeroneCAS# 10162-99-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 10162-99-9 | SDF | Download SDF |
| PubChem ID | 139292108.0 | Appearance | Powder |
| Formula | C21H30O5 | M.Wt | 362.47 |
| Type of Compound | Steroids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (10R,13R,14S)-17-acetyl-2,3,14-trihydroxy-10,13-dimethyl-2,3,4,5,9,11,12,15,16,17-decahydro-1H-cyclopenta[a]phenanthren-6-one | ||
| SMILES | CC(=O)C1CCC2(C1(CCC3C2=CC(=O)C4C3(CC(C(C4)O)O)C)C)O | ||
| Standard InChIKey | VNLQNGYIXVTQRR-BLMSIPRSSA-N | ||
| Standard InChI | InChI=1S/C21H30O5/c1-11(22)12-5-7-21(26)14-8-16(23)15-9-17(24)18(25)10-19(15,2)13(14)4-6-20(12,21)3/h8,12-13,15,17-18,24-26H,4-7,9-10H2,1-3H3/t12?,13?,15?,17?,18?,19-,20-,21-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Poststerone Dilution Calculator

Poststerone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7588 mL | 13.7942 mL | 27.5885 mL | 55.177 mL | 68.9712 mL |
| 5 mM | 0.5518 mL | 2.7588 mL | 5.5177 mL | 11.0354 mL | 13.7942 mL |
| 10 mM | 0.2759 mL | 1.3794 mL | 2.7588 mL | 5.5177 mL | 6.8971 mL |
| 50 mM | 0.0552 mL | 0.2759 mL | 0.5518 mL | 1.1035 mL | 1.3794 mL |
| 100 mM | 0.0276 mL | 0.1379 mL | 0.2759 mL | 0.5518 mL | 0.6897 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Lyciumin B
Catalog No.:BCX1303
CAS No.:125756-66-3
- Theasaponin E1
Catalog No.:BCX1302
CAS No.:220114-28-3
- Kakkanin
Catalog No.:BCX1301
CAS No.:63770-91-2
- 16α-hydroxy-3-oxo-lanosta-7,9(11),24-trien-21-oic acid
Catalog No.:BCX1300
CAS No.:862109-64-6
- Xylopine
Catalog No.:BCX1299
CAS No.:517-71-5
- Lyciumin A
Catalog No.:BCX1298
CAS No.:125708-06-7
- Kanshone C
Catalog No.:BCX1297
CAS No.:117634-64-7
- Hypoglaunine D
Catalog No.:BCX1296
CAS No.:220751-00-8
- Amaroswerin
Catalog No.:BCX1295
CAS No.:21233-18-1
- t-OMe-Byakangelicin
Catalog No.:BCX1294
CAS No.:79638-04-3
- Ajugasterone C 2-acetate
Catalog No.:BCX1293
CAS No.:154510-93-7
- Chaparrinone
Catalog No.:BCX1292
CAS No.:22611-34-3
- Corymbiferin
Catalog No.:BCX1305
CAS No.:5042-09-1
- Shinjulactone M
Catalog No.:BCX1306
CAS No.:103630-27-9
- 13α,21-Dihydroeurycomanone
Catalog No.:BCX1307
CAS No.:129587-06-0
- Entadamide A
Catalog No.:BCX1308
CAS No.:100477-88-1
- 3'-O-Angeloylhamaudol
Catalog No.:BCX1309
CAS No.:84272-84-4
- Theasaponin E2
Catalog No.:BCX1310
CAS No.:220114-30-7
- Oxypeucedanin hydrate-3”-ethyl ether
Catalog No.:BCX1311
CAS No.:55481-87-3
- Isooxypeucedanin
Catalog No.:BCX1312
CAS No.:5058-15-1
- Poricoic acid BM
Catalog No.:BCX1313
CAS No.:1815623-74-5
- Neoartanin
Catalog No.:BCX1314
CAS No.:104196-69-2
- Rabdosia acid A
Catalog No.:BCX1315
CAS No.:1884697-13-5
- Tunicoidine A
Catalog No.:BCX1316
CAS No.:1415979-39-3
Quinoa as phytopharmaceutical? Urinary elimination of ecdysterone after consumption of quinoa alone and in combination with spinach.[Pubmed:38400693]
Arch Pharm (Weinheim). 2024 Feb 24:e2300689.
The phytosteroid ecdysterone is classified as an anabolic agent and has been included on the monitoring list of the World Anti-Doping Agency since 2020. Therefore, the consumption of food rich in ecdysterone, such as quinoa and spinach, is the focus of a lively debate. Thus, the urinary excretion of ecdysterone and its metabolites in humans was investigated following quinoa consumption alone and in combination with spinach. Eight participants (four male and four female) were included, and they ingested 368 +/- 61 g cooked quinoa alone and in combination with 809 +/- 115 g spinach after a washout. Post-administration urines were analyzed by LC-MS/MS. After intake of both preparations, ecdysterone and two metabolites were excreted in the urine. The maximum concentration of ecdysterone ranged from 0.44 to 5.5 microg/mL after quinoa and from 0.34 to 4.1 microg/mL after quinoa with spinach. The total urinary excreted amount as parent drug plus metabolites was 2.61 +/- 1.1% following quinoa intake and 1.7 +/- 0.9% in combination with spinach. Significant differences were found in the total urinary excreted amount of ecdysterone, 14-deoxy-ecdysterone, and 14-deoxy-Poststerone. Only small portions of ecdysterone from quinoa and the combination with spinach were excreted in the urine, suggesting that both quinoa and spinach are poor sources of ecdysterone in terms of bioavailability.
Urinary Excretion of Ecdysterone and Its Metabolites Following Spinach Consumption.[Pubmed:37161586]
Mol Nutr Food Res. 2023 Jul;67(14):e2200518.
SCOPE: The phytosteroid ecdysterone is present in spinach. In this study, the urinary elimination of ecdysterone and its metabolites in humans is investigated following spinach consumption of two different culinary preparations. METHODS AND RESULTS: Eight participants (four males, four females) ingested 950 (27.1) g sauteed spinach (average [+/-standard deviation (SD)]) and 912 (70.6) g spinach smoothie as second intervention after washout. Post-administration urines are analyzed by liquid chromatography tandem mass spectrometry (LC-MS/MS). After intake of both preparations, ecdysterone and two metabolites, 14-deoxy-ecdysterone, and 14-deoxy-Poststerone, are excreted in urine. The maximum concentration of ecdysterone is ranging from 0.09 to 0.41 microg mL(-1) after sauteed spinach and 0.08-0.74 microg mL(-1) after smoothie ingestion. The total excreted amount (mean% [+/-SD]) in the urine as a parent drug plus the metabolites is only 1.4 (1.0) for both sauteed spinach and smoothie. The apparent sex related differences in 14-deoxy-Poststerone excretion will need further investigations. CONCLUSION: Only a small proportion of ecdysterone from spinach is excreted into urine. No significant differences are found in concentration and recovered amount (%) of ecdysterone, 14-deoxy-ecdysterone, and 14-deoxy-Poststerone in urine between sauteed spinach and smoothie ingestion. A discrimination between ecdysterone from food or preparations will be challenging based on urinary concentrations only, at least for later post-administration samples.
Comprehensive metabolic profiles of Achyranthes bidentate in rat serum via ultra-high performance liquid chromatography time-of-flight mass spectrometry and their correlation with osteoinductive activity.[Pubmed:37116317]
J Pharm Biomed Anal. 2023 Jul 5;231:115418.
The osteoinductive effect of crude and salt-processed Achyranthes bidentata is associated with the serum metabolites. Grey relationship analysis between the serum metabolites and osteoinductive effect will help to clarify the bioactive serum metabolites. First, an ultra-high performance liquid chromatography time-of-flight mass spectrometry method was used to develop serum metabolic fingerprint of rats after oral administration of crude and salt-processed Achyranthes bidentata. The MS(1) and MS(2) data of serum metabolites were scanned in the range of m/z 100-1500 and 50-1200, respectively. The chemical structures of the metabolites were thoroughly elucidated. Two prototypes and twelve metabolites have been identified. Second, osteoblasts were cultured with the drug-containing serum at different time points. The osteoinductive effect of crude and salt-processed Achyranthes bidentata was evaluated by detecting the proliferation rate and alkaline phosphatase activity of osteoblasts. Third, grey correlation analysis was utilized to elucidate the spectral-effect relationship between serum metabolic fingerprints and osteoinductive effect. Finally, the correlation coefficients of ten metabolites, i.e., oleanolic acid, Poststerone-M1, chikusetsusaponin V-M1, oleanolic acid-M2, oleanolic acid-M4, spinacoside D-M1, chikusetsusaponin I-M1, betavulgaroside IV-M2, chikusetsusaponin IVa and achyranthoside IV-M1 were above 0.7. Collectively, our work will provide helpful knowledge for the future research on Achyranthes bidentata.
Urinary Elimination of Ecdysterone and Its Metabolites Following a Single-Dose Administration in Humans.[Pubmed:34207569]
Metabolites. 2021 Jun 9;11(6):366.
Ecdysterone is a phytosteroid widely discussed for its various pharmacological, growth-promoting, and anabolic effects, mediated by the activation of estrogen receptor beta (ERbeta). Performance-enhancement in sports was demonstrated recently, and ecdysterone was consequently included in the Monitoring Program, to detect potential patterns of misuse in sport. Only few studies on the pharmacokinetics of ecdysterone in humans have been reported so far. In this study, post-administration urine samples in twelve volunteers (single dose of 50 mg of ecdysterone) were analyzed using dilute-and-inject liquid-chromatography-tandem mass spectrometry. Identification and quantitation of ecdysterone and of two metabolites, 14-deoxy-ecdysterone and 14-deoxy-Poststerone, was achieved. Ecdysterone was the most abundant analyte present in post-administration urine samples, detected for more than 2 days, with a maximum concentration (C(max)) in the 2.8-8.5 h urine (C(max) = 4.4-30.0 microg/mL). The metabolites 14-deoxy-ecdysterone and 14-deoxy-Poststerone were detected later, reaching the maximum concentrations at 8.5-39.5 h (C(max) = 0.1-6.0 microg/mL) and 23.3-41.3 h (C(max) = 0.1-1.5 microg/mL), respectively. Sex-specific differences were not observed. Cumulative urinary excretion yielded average values of 18%, 2.3%, and 1.5% for ecdysterone, 14-deoxy-ecdysterone, and 14-deoxy-Poststerone, respectively. Ecdysterone and 14-deoxy-ecdysterone were excreted following first-order kinetics with half-lives calculated with three hours, while pharmacokinetics of 14-deoxy-Poststerone needs further evaluation.
The complex metabolism of poststerone in male rats.[Pubmed:33862260]
J Steroid Biochem Mol Biol. 2021 Sep;212:105897.
Ecdysteroids are not endogenous to mammals, but are normal components of the food intake of many mammalian species consuming phytoecdysteroid-containing plants. The most frequently encountered phytoecdysteroid is 20-hydroxyecdysone (20E). Several pharmaceutical effects have been observed after ecdysteroid injection or ingestion, but it is not clear to what extent metabolites generated in the mammalian body contribute to these effects. The C21-ecdysteroid Poststerone (Post) is a metabolite of 20E in rodents. Post analogues are key intermediates in the metabolism of exogenous ecdysteroids possessing a C20/22-diol. The pharmacokinetics, bioavailability and metabolism of Post have been assessed in male rats after ingestion and injection. The bioavailability of Post is significantly greater than that of 20E and the presence of an efficient entero-hepatic cycle allows Post to be effectively metabolised to a wide range of metabolites which are excreted mainly in the faeces, but also to some extent in the urine. Several of the major metabolites in the bile have been identified unambiguously as 3-epi-Poststerone, 16alpha-hydroxyPoststerone, 21-hydroxyPoststerone and 3-epi-21-hydroxyPoststerone. Conjugates are also present. Parallels are drawn to the metabolism of endogenous vertebrate steroid hormones, to which Post bears more similarity than 20E.
Ecdysteroid metabolism in mammals: The fate of ingested 20-hydroxyecdysone in mice and rats.[Pubmed:33819630]
J Steroid Biochem Mol Biol. 2021 Sep;212:105896.
Phytoecdysteroids are molecules derived from sterol metabolism and found in many plants. They display a wide array of pharmacological effects on mammals (e.g. anabolic, anti-diabetic). Although these effects have been long established, the molecular targets involved remain to be identified. Like endogenous steroid hormones and bile acids, which are biochemically related, ingested or injected phytoecdysteroids undergo a set of reactions in mammals leading to the formation of numerous metabolites, only some of which have been so far identified, and it is presently unknown whether they represent active metabolites or inactivation products. In the large intestine, ecdysteroids undergo efficient 14-dehydroxylation. Other changes (reductions, epimerization, side-chain cleavage) are also observed, but whether these occur in the liver and/or large intestine is not known. The purpose of this study was to investigate the pharmacokinetics of 20-hydroxyecdysone (20E), the most common phytoecdysteroid, when administered to mice and rats, using, when required, tritium-labelled molecules to permit metabolic tracking. Bioavailability, the distribution of radioactivity and the kinetics of formation of metabolites were followed for 24-48 hours after ingestion and qualitative and quantitative analyses of circulating and excreted compounds were performed. In mice, the digestive tract always contains the majority of the ingested 20E. Within 30 min after ingestion, 20E reaches the large intestine, where microorganisms firstly remove the 14-hydroxyl group and reduce the 6-one. Then a very complex set of metabolites (not all of which have yet been identified) appears, which correspond to Poststerone derivatives formed in the liver. We have observed that these compounds (like bile acids) undergo an entero-hepatic cycle, involving glucuronide conjugation in the liver and subsequent deconjugation in the intestine. Despite the very short half-life of ecdysteroids in mammals, this entero-hepatic cycle helps to maintain their plasma levels at values which, albeit low (Poststerone and 14-deoxyPoststerone and their diverse reduction products; the major products of this metabolism have been unambiguously identified. The major sites of metabolism of exogenous ecdysteroids in mammals are the large intestine and the liver. The entero-hepatic cycle contributes to the metabolism and to maintaining a low, but pharmacologically significant, concentration of ecdysteroids in the blood for ca. 24 h after ingestion. These data, together with parallel in vitro experiments provide a basis for the identification of 20E metabolite(s) possibly involved in the physiological effects associated with ecdysteroids in mammals.
In vitro adjuvant antitumor activity of various classes of semi-synthetic poststerone derivatives.[Pubmed:33261846]
Bioorg Chem. 2021 Jan;106:104485.
Various classes of semi-synthetic analogs of Poststerone, the product of oxidative cleavage of the C20-C22 bond in the side chain of the phytoecdysteroid 20-hydroxyecdysone, were synthesized. The analogs were obtained by reductive transformations using L-Selectride and H(2)-Pd/C, by molecular abeo-rearrangements using the DAST reagent or ultrasonic treatment in the NaI-Zn-DMF system, and by acid-catalyzed reactions of Poststerone derivatives with various aldehydes (o-FC(6)H(4)CHO, m-CF(3)C(6)H(4)CHO, CO(2)Me(CH(2))(8)CHO). The products were tested on a mouse lymphoma cell line pair, L5178 and its ABCB1-transfected multi-drug resistant counterpart, L5178(MDR), for their in vitro activity alone and in combination with doxorubicin, and for the ability to inhibit the ABCB1 transporter. Among the tested compounds, new 2,3-dioxolane derivatives of the pregnane ecdysteroid were found to have a pronounced chemosensitizing activity towards doxorubicin and could be considered as promising candidates for further structure optimization for the development of effective chemosensitizing agents.
Molecular rearrangements of poststerone derivative steroid core with formation of unique D-homostructures of pregnane and androstane series.[Pubmed:31075339]
Steroids. 2019 Aug;148:28-35.
20R-Hydroxy short-chain ecdysteroids were synthesized by chemo- and stereoselective reduction of Poststerone acetonide with L-Selectride or LiAlH(4). The same reaction with the excess of L- Selectride followed by the treatment of the reaction mixture with hydrochloric acid is accompanied by (8R)-13(14 --> 8)abeo- rearrangements, which resulted in the contraction/expansion of C/D pregnane rings. The reaction of 20R-hydroxy Poststerone analogs with (diethylamino)sulfur trifluoride (DAST) proceeds through intramolecular rearrangements and provides D-homo- or 13,14-seco- androstane structures.
Poststerone increases muscle fibre size partly similar to its metabolically parent compound, 20-hydroxyecdysone.[Pubmed:30923008]
Fitoterapia. 2019 Apr;134:459-464.
In mice, Poststerone is a major in vivo metabolite of the worldwide popular anabolic food supplement 20-hydroxyecdysone (20E). Here we present the first study on this ecdysteroid in view of the in vivo anabolic effect of its parent compound, 20E in mammals. We have monitored muscle fibre type cross sectional areas (CSA) of developing rats after treatment with Poststerone as we did in a previous study with 20E. The muscle mass and fibre CSAs of soleus and EDL were increased by Poststerone in a muscle specific manner as by 20E but there were some differences. Notably, the CSAs of type I and type IIa fibres in the soleus were less elevated by Poststerone than by 20E. However Poststerone increased the CSA of each four fibre types (I, IIa, IIx, IIb) in the EDL more effectively than 20E did. Poststerone, like 20E, also increased the number of myonuclei in the EDL of both hind limbs. Overall, this shows for the first time that Poststerone having steroid nucleus and no side chain of 20E has a partly overlapping effect with that of 20E.
Sonochemically assisted 2,3-dideoxygenation and skeletal rearrangement of ecdysteroid derivatives.[Pubmed:30594517]
Ultrason Sonochem. 2019 Apr;52:505-511.
Sonochemical 2,3-dideoxygenation of ecdysteroids with the Delta (2(3))-bond generation and activation of the C-C bonds of the steroid core in the Poststerone derivatives, that causes the skeletal rearrangement have been carried out for the first time. Thus, the ultrasonically assisted reaction of 2,3-dimesyloxy derivatives of ecdysteroids with the NaI-Zn-DMF system gives rise to their 2,3-dideoxy-Delta(2(3))-analogues with yields 70-92%. In the case of 2,3-dimesyloxyPoststerone as the initial ecdysteroid substrate the reaction is accompanied by the activation of the allyl moiety and semipinacol rearrangement, resulting in the C(13)-C(14) bond migration with C/D rings contraction/expansion and providing novel short chain (8R)-13(14 --> 8)-abeo-isomer.
Side-chain cleaved phytoecdysteroid metabolites as activators of protein kinase B.[Pubmed:30428419]
Bioorg Chem. 2019 Feb;82:405-413.
Phytoecdysteroids exert their non-hormonal anabolic and adaptogenic effects in mammals, including humans, through a partially revealed mechanism of action involving the activation of protein kinase B (Akt). We have recently found that Poststerone, a side-chain cleaved in vivo metabolite of 20-hydroxyecdysone, exerts potent anabolic activity in rats. Here we report the semi-synthetic preparation of a series of side-chain cleaved ecdysteroids and their activity on the Akt phosphorylation in murine skeletal muscle cells. Twelve C-21 ecdysteroids including 8 new compounds were obtained through the oxidative side-chain cleavage of various phytoecdysteroids, or through the base-catalyzed autoxidation of Poststerone. The complete (1)H and (13)C NMR spectroscopic assignments of the new compounds are presented. Among the tested compounds, 9 could activate Akt stronger than Poststerone revealing that side-chain cleaved derivatives of phytoecdysteroids other than 20-hydroxyecdysone are valuable bioactive metabolites. Thus, our results suggest that the expectable in vivo formation of such compounds should contribute to the bioactivity of herbal preparations containing ecdysteroid mixtures.
Backstabbing P-gp: Side-Chain Cleaved Ecdysteroid 2,3-Dioxolanes Hyper-Sensitize MDR Cancer Cells to Doxorubicin without Efflux Inhibition.[Pubmed:28125071]
Molecules. 2017 Jan 25;22(2):199.
P-glycoprotein (P-gp, ABCB1) over-expression, causing a multi-drug resistant (MDR) phenotype, is a major problem in cancer chemotherapy that urgently requires novel approaches. Our previous studies showed certain ecdysteroid derivatives as promising chemo-sensitizers against MDR and non-MDR cancer cell lines while also exerting mild to moderate inhibition of P-gp function. Here we report the preparation of a set of substituted 2,3-dioxolane derivatives of Poststerone, a known in vivo metabolite of 20-hydroxyecdysone (20E). In contrast with previously studied ecdysteroid dioxolanes, the majority of the new compounds did not inhibit the efflux function of P-gp. Nevertheless, a strong, dose dependent sensitization to doxorubicin was observed on a P-gp transfected cancer cell line and on its susceptible counterpart. We also observed that the MDR cell line was more sensitive to the compounds' effect than the non-MDR. Our results showed for the first time that the chemo-sensitizing activity of ecdysteroids can be fully independent of functional efflux pump inhibition, and suggest these compounds as favorable leads against MDR cancer.
The metabolism of 20-hydroxyecdysone in mice: relevance to pharmacological effects and gene switch applications of ecdysteroids.[Pubmed:21439380]
J Steroid Biochem Mol Biol. 2011 Aug;126(1-2):1-9.
Ecdysteroids exert many pharmacological effects in mammals (including humans), most of which appear beneficial, but their mechanism of action is far from understood. Whether they act directly and/or after the formation of metabolites is still an open question. The need to investigate this question has gained extra impetus because of the recent development of ecdysteroid-based gene-therapy systems for mammals. In order to investigate the metabolic fate of ecdysteroids in mice, [1alpha,2alpha-(3)H]20-hydroxyecdysone was prepared and injected intraperitoneally to mice. Their excretory products (urine+faeces) were collected and the different tritiated metabolites were isolated and identified. The pattern of ecdysteroid metabolites is very complex, but no conjugates were found, in contrast to the classical fate of the (less polar) endogenous vertebrate steroid hormones. Primary reactions involve dehydroxylation at C-14 and side-chain cleavage between C-20 and C-22, thereby yielding 14-deoxy-20-hydroxyecdysone, Poststerone and 14-deoxyPoststerone. These metabolites then undergo several reactions of reduction involving, in particular, the 6-keto-group. A novel major metabolite has been identified as 2beta,3beta,6alpha,22R,25-pentahydroxy-5beta-cholest-8(14)-ene. The formation of this and the other major metabolites is discussed in relation to the various effects of ecdysteroids already demonstrated on vertebrates.
Ecdysteroids from Cyanotis longifolia Benth. (Commelinaceae).[Pubmed:19760659]
Arch Insect Biochem Physiol. 2009 Dec;72(4):194-209.
Cyanotis longifolia Benth. (Commelinaceae) contains ecdysteroids, which are highly concentrated in the roots and flowers, whereas leaves contain only very low amounts and stems intermediate amounts. 20-Hydroxyecdysone is the major component found in all tissues, but roots also contain large amounts of 20-hydroxyecdysone 3-acetate and ajugasterone C. A preparative experiment has shown that roots contain a complex ecdysteroid mixture, and the analysis of minor components has allowed the isolation of several already known ecdysteroids (polypodine B, 2-deoxy-20,26-dihydroxyecdysone, isovitexirone, Poststerone) together with five new (ajugasterone C 3-acetate, 5beta-hydroxy-Poststerone, Poststerone 2-acetate, 14(15)-dehydro-Poststerone 2-acetate, 24-epi-atrotosterone A [=24-methyl-ajugasterone C]) ecdysteroids that have been fully characterized. A preliminary investigation of 55 species belonging to 15 different genera of the Commelinaceae has shown that several of them contain significant concentrations of ecdysteroids, among which some previously uninvestigated ones appear to be very promising sources of ecdysteroids.


