XylopineCAS# 517-71-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 517-71-5 | SDF | Download SDF |
| PubChem ID | 160503.0 | Appearance | Powder |
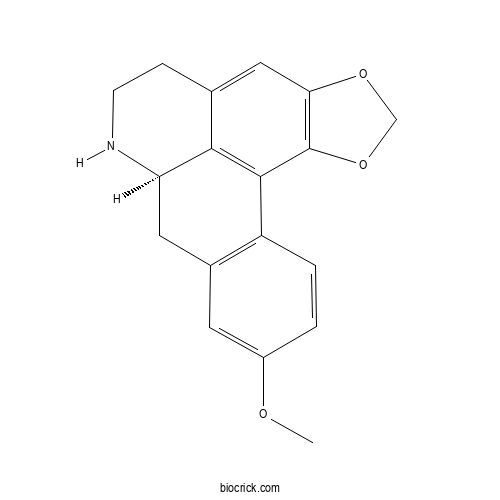
| Formula | C18H17NO3 | M.Wt | 295.34 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (12R)-16-methoxy-3,5-dioxa-11-azapentacyclo[10.7.1.02,6.08,20.014,19]icosa-1(20),2(6),7,14(19),15,17-hexaene | ||
| SMILES | COC1=CC2=C(C=C1)C3=C4C(C2)NCCC4=CC5=C3OCO5 | ||
| Standard InChIKey | RFWCCZDSXIZJMF-CQSZACIVSA-N | ||
| Standard InChI | InChI=1S/C18H17NO3/c1-20-12-2-3-13-11(6-12)7-14-16-10(4-5-19-14)8-15-18(17(13)16)22-9-21-15/h2-3,6,8,14,19H,4-5,7,9H2,1H3/t14-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Xylopine Dilution Calculator

Xylopine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.3859 mL | 16.9296 mL | 33.8593 mL | 67.7186 mL | 84.6482 mL |
| 5 mM | 0.6772 mL | 3.3859 mL | 6.7719 mL | 13.5437 mL | 16.9296 mL |
| 10 mM | 0.3386 mL | 1.693 mL | 3.3859 mL | 6.7719 mL | 8.4648 mL |
| 50 mM | 0.0677 mL | 0.3386 mL | 0.6772 mL | 1.3544 mL | 1.693 mL |
| 100 mM | 0.0339 mL | 0.1693 mL | 0.3386 mL | 0.6772 mL | 0.8465 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Lyciumin A
Catalog No.:BCX1298
CAS No.:125708-06-7
- Kanshone C
Catalog No.:BCX1297
CAS No.:117634-64-7
- Hypoglaunine D
Catalog No.:BCX1296
CAS No.:220751-00-8
- Amaroswerin
Catalog No.:BCX1295
CAS No.:21233-18-1
- t-OMe-Byakangelicin
Catalog No.:BCX1294
CAS No.:79638-04-3
- Ajugasterone C 2-acetate
Catalog No.:BCX1293
CAS No.:154510-93-7
- Chaparrinone
Catalog No.:BCX1292
CAS No.:22611-34-3
- Physcion-8-O-β-gentiobioside
Catalog No.:BCX1291
CAS No.:84268-38-2
- Wistin
Catalog No.:BCX1290
CAS No.:19046-26-5
- Limocitrin 3-O-β-D-glucopyranoside
Catalog No.:BCX1289
CAS No.:38836-51-0
- Ajugose
Catalog No.:BCX1288
CAS No.:512-72-1
- Dihydrocatalpol
Catalog No.:BCX1287
CAS No.:6736-86-3
- 16α-hydroxy-3-oxo-lanosta-7,9(11),24-trien-21-oic acid
Catalog No.:BCX1300
CAS No.:862109-64-6
- Kakkanin
Catalog No.:BCX1301
CAS No.:63770-91-2
- Theasaponin E1
Catalog No.:BCX1302
CAS No.:220114-28-3
- Lyciumin B
Catalog No.:BCX1303
CAS No.:125756-66-3
- Poststerone
Catalog No.:BCX1304
CAS No.:10162-99-9
- Corymbiferin
Catalog No.:BCX1305
CAS No.:5042-09-1
- Shinjulactone M
Catalog No.:BCX1306
CAS No.:103630-27-9
- 13α,21-Dihydroeurycomanone
Catalog No.:BCX1307
CAS No.:129587-06-0
- Entadamide A
Catalog No.:BCX1308
CAS No.:100477-88-1
- 3'-O-Angeloylhamaudol
Catalog No.:BCX1309
CAS No.:84272-84-4
- Theasaponin E2
Catalog No.:BCX1310
CAS No.:220114-30-7
- Oxypeucedanin hydrate-3”-ethyl ether
Catalog No.:BCX1311
CAS No.:55481-87-3
Computational studies on potential small molecule inhibitors of Leishmania pteridine reductase 1.[Pubmed:36632757]
J Biomol Struct Dyn. 2023;41(21):12128-12141.
Leishmaniasis is a neglected tropical disease of major public health concern. Challenges with current therapeutics have led to the exploration of plant medicine for potential antileishmanial agents. Despite the promising activity of some antileishmanial natural products, their protein targets have not been explored. The relevance of folate metabolism in the Leishmania parasite's existence presents crucial targets for the development of antileishmanial chemotherapy. Pteridine reductase 1 (PTR1), a crucial enzyme involved in DNA biosynthesis, is a validated target of the Leishmania parasite. Unearthing inhibitors of this enzyme is therefore an active research area. The goal of this work is to unearth small molecule inhibitors of PTR1 using molecular docking and molecular dynamic simulations. Thus, the interactions between selected antileishmanial natural products and PTR1 were examined. The binding affinities obtained from molecular docking ranged from -6.2 to -9.8 kcal/mol. When compared to the natural PTR1 substrate biopterin, compounds such as anonaine, chimanine D, corynantheine, grifolin, licochalcone A, piperogalin and Xylopine produced better binding affinities, making interactions catalytic residues - Tyr194, Asp181, Phe113, Arg17 and Ser111. The PTR1- Xylopine, -piperogalin, -grifolin, and -licochalcone A complexes exhibited remarkable stability under dynamic conditions during the entire 200 ns simulation period. The overall binding free energy of grifolin, piperogalin, and licochalcone A were observed to be -105.711, -103.567, and -105.646 kJ/mol respectively. The binding of these complexes was observed to be favorable and spontaneous and as such capable of inhibiting Leishmania PTR1. They could therefore be considered as candidates in the development of antileishmanial chemotherapy.Communicated by Ramaswamy H. Sarma.
Anonazepine, a new alkaloid from the leaves of Annona muricata (Annonaceae).[Pubmed:36544263]
Z Naturforsch C J Biosci. 2022 Dec 22;78(5-6):247-251.
From the CHCl(3)-soluble extract of Annona muricata L. (Annonaceae) leaves, one new 3-benzazepine-type alkaloid, anonazepine (1), and four known aporphine-type alkaloids, (+)-laurotetanine (2), (+)-norglaucine (3), (-)-Xylopine (4), and lanuginosine (5), were isolated. Except for (-)-Xylopine (4), these remaining known alkaloids were first reported in A. muricata. The structures of the isolated alkaloids were established by 1D and 2D NMR spectroscopy and MS, as well as comparison with literature data. The new 3-benzazepine-type alkaloid existed in an inseparable mixture of two equilibrium conformers. Its absolute configuration was determined based on comparing their experimental and calculated ECD data. The anti-inflammatory activity of the isolated alkaloids was investigated, but none of the alkaloids showed a significant result.
Antifungal Annona muricata L. (soursop) extract targets the cell envelope of multi-drug resistant Candida albicans.[Pubmed:36280018]
J Ethnopharmacol. 2023 Jan 30;301:115856.
ETNOPHARMACOLOGICAL RELEVANCE: Annona muricata L. (soursop) is traditionally used in the treatment of inflammatory diseases, cancer, and infections caused by fungi. The therapeutic activity explored by its medicinal use is generally associated with its phytoconstituents, such as acetogenins and alkaloids. However, its potential antifungal bioactivity as well as its mechanism of action remains to be established. AIM OF THE STUDY: To evaluate the antifungal activity of the ethanolic extract of A. muricata leaves against multidrug-resistant Candida albicans (ATCC(R) 10231). MATERIAL AND METHODS: Phytoconstituents were detected by UFLC-QTOF-MS. The minimum inhibitory concentration was determined, followed by the determination of the minimum fungicidal concentration. For planktonic cells, the growth curve and cell density were evaluated. Studies to understand the mechanism of action on the cell envelope involved crystal violet permeability, membrane extravasation, sorbitol protection, exogenous ergosterol binding assay, metabolic activity, and cell viability. Furthermore, mitochondrial membrane potential was assessed. RESULTS: Our analyses demonstrated a significant inhibitory effect of A. muricata, with the ability to reduce fungal growth by 58% and cell density by 65%. The extract affected both the fungal plasma membrane and cell wall integrity, with significant reduction of the cell viability. Depolarization of the fungal mitochondrial membrane was observed after treatment with A. muricata. Rutin, xi-anomuricine, kaempferol-3O-rutinoside, nornuciferine, Xylopine, atherosperminine, caffeic acid, asimilobine, s-norcorydine, loliolide, annohexocin, annomuricin, annopentocin, and sucrose were identified as extract bioactive components. CONCLUSIONS: Our findings show that the A. muricata extract is a source of chemical diversity, which acts as a potential antifungal agent with promising application to the therapy of infections caused by C. albicans.
Phytochemical Analysis of the Fruit Pulp Extracts from Annona crassiflora Mart. and Evaluation of Their Antioxidant and Antiproliferative Activities.[Pubmed:35885322]
Foods. 2022 Jul 13;11(14):2079.
Annona crassiflora Mart., the marolo fruit of the Cerrado biome, is one of the most frequently consumed species from the Brazilian Midwest. This study aimed to evaluate the chemical composition and the antioxidant and cytotoxic properties of the fruit pulp of A. crassiflora collected at Chapada das Mesas, Maranhao, Brazil. The volatile concentrate was identified as mainly ethyl octanoate, ethyl hexanoate, and methyl octanoate. From the ethanol (LFP-E) and ethyl acetate (LFP-A) extracts were identified phenolic acids (p-coumaric, gallic, quinic, and ferulic), flavones and derivatives (apigenin, epicatechin, 2'-5-dimethoxyflavone, 3',7-dimethoxy-3-hydroxyflavone, kaempferol-3-O-glucoside and 3-O-rutinoside, quercetin-3-O-glucoside, procyanidin B2, and rutin), aporphine alkaloids (Xylopine, stephagine, and romucosine), and acetogenin (annonacin). For the LFP-E and LFP-A extracts, the total phenolic compound values were 15.89 and 33.16 mg GAE/g, the flavonoid compound content values were 2.53 and 70.55 mg QE/g, the DPPH radical scavenging activity showed EC(50) values of 182.54 and 57.80 microg/mL, and the ABTS radical activity showed TEAC values of 94.66 and 192.61 microM TE/g. The LFP-E extract showed significant cytotoxicity and cell selectivity for the U251-glioma strain, presenting a GI(50) value of 21.34 microg/mL, which is close to doxorubicin (11.68 microg/mL), the standard chemotherapeutic drug. The marolo fruit seems to be a promising source for developing innovative and healthy products for the food industry.
Alkaloids from Phaeanthus vietnamensis with inhibitory effect on nitric oxide production lipopolysaccharide-stimulated in RAW264.7 macrophages.[Pubmed:34779313]
J Asian Nat Prod Res. 2022 Sep;24(9):898-903.
The chemical study of the acidic extract of Phaeanthus vietnamensis leaves led to the isolation of one new alkaloid, vietnamine A (1) and eight known alkaloids (R,S)-2N-norberbamunine (2), grisabine (3), 1S,1'R,O,O'-dimethylgrisabine (4), dauricine (5), neothalibrine (6), vietnamine (7), Xylopine (8), and argentinine (9) by NMR and MS and comparing with the data reported in the literature. Compounds 1-9 were evaluated for inhibitory NO production in RAW 264.7 macrophages, LPS-stimulated. Compounds 1-3 significantly inhibited on NO production with the IC(50) values of 6.8 +/- 0.9, 9.8 +/- 1.0, and 7.1 +/- 0.4 microg/ml, respectively.
Natural Aporphine Alkaloids with Potential to Impact Metabolic Syndrome.[Pubmed:34684698]
Molecules. 2021 Oct 10;26(20):6117.
The incidence and prevalence of metabolic syndrome has steadily increased worldwide. As a major risk factor for various diseases, metabolic syndrome has come into focus in recent years. Some natural aporphine alkaloids are very promising agents in the prevention and treatment of metabolic syndrome and its components because of their wide variety of biological activities. These natural aporphine alkaloids have protective effects on the different risk factors characterizing metabolic syndrome. In this review, we highlight the activities of bioactive aporphine alkaloids: thaliporphine, boldine, nuciferine, pronuciferine, roemerine, dicentrine, magnoflorine, anonaine, apomorphine, glaucine, predicentrine, isolaureline, Xylopine, methylbulbocapnine, and crebanine. We particularly focused on their impact on metabolic syndrome and its components, including insulin resistance and type 2 diabetes mellitus, endothelial dysfunction, hypertension and cardiovascular disease, hyperlipidemia and obesity, non-alcoholic fatty liver disease, hyperuricemia and kidney damage, erectile dysfunction, central nervous system-related disorder, and intestinal microbiota dysbiosis. We also discussed the potential mechanisms of actions by aporphine alkaloids in metabolic syndrome.
Anti-Infective and Anti-Cancer Properties of the Annona Species: Their Ethnomedicinal Uses, Alkaloid Diversity, and Pharmacological Activities.[Pubmed:31816948]
Molecules. 2019 Dec 3;24(23):4419.
Annona species have been a valuable source of anti-infective and anticancer agents. However, only limited evaluations of their alkaloids have been carried out. This review collates and evaluates the biological data from extracts and purified isolates for their anti-infective and anti-cancer activities. An isoquinoline backbone is a major structural alkaloid moiety of the Annona genus, and more than 83 alkaloids have been isolated from this genus alone. Crude extracts of Annona genus are reported with moderate activities against Plasmodium falciparum showing larvicidal activities. However, no pure compounds from the Annona genus were tested against the parasite. The methanol extract of Annona muricata showed apparent antimicrobial activities. The isolated alkaloids from this genus including liriodenine, anonaine, asimilobine showed sensitivity against Staphylococcus epidermidis. Other alkaloids such as (+)-Xylopine and isocoreximine indicated significant anti-cancer activity against A549 and K-562 cell lines, respectively. This review revealed that the alkaloids from Annona genus are rich in structural diversity and pharmacological activities. Further exploration of this genus and their alkaloids has potential for developing novel anti-infective and anticancer drugs.
Alkaloids from the root of Indonesian Annona muricata L.[Pubmed:31282747]
Nat Prod Res. 2021 Feb;35(3):481-489.
Annona muricata L. has been used traditionally in Indonesia to treat disease. Phytochemical studies on the alkaloid fractions from the root of Annona muricata L. from Malang-Indonesia resulted in the isolation of an unreported benzylisoquinoline alkaloid (+)-Xylopine 5 as well as four known alkaloids (1-4). The crude methanol extract and alkaloid fractions were tested against Plasmodium falciparum K1 and against bacteria (Escherichia coli, Klebsiella pneumonia, Acinetobacter buamanii, Pseudomonas aeruginosa, Methicillin-resistant Staphylococcus aureus) with insignificant activities (MIC > 32 microg/mL). Individual alkaloids were tested against a human suspension cancer cell line (HL-60 leukemia cells) and two human fibroblastic cancer cell lines (A549 lung cancer cells and HepG2 liver cancer cells) in which compound 5 was the most toxic alkaloid with IC(50) values ranging from 20 to 80 microM.
Detection and identification of acetylcholinesterase inhibitors in Annona cherimola Mill. by effect-directed analysis using thin-layer chromatography-bioassay-mass spectrometry.[Pubmed:31183917]
Phytochem Anal. 2019 Nov;30(6):679-686.
INTRODUCTION: Acetylcholinesterase (AChE) inhibitors are considered an important strategy in the treatment of neurological disorders such as Alzheimer's disease. A simple and fast planar chromatography-bioassay methodology has been established to detect bioactive molecules in cherimoya fruit. OBJECTIVE: Detect and identify AChE inhibitors in cherimoya by high-performance thin-layer chromatography (HPTLC)-bioassay-mass spectrometry (MS) and related techniques. METHODOLOGY: Effect-directed analysis by planar chromatography-bioassay-mass spectrometry was applied to detect and identify AChE inhibitors in pulp, peel and cherimoya seed. Bioassay was optimised establishing the following conditions: enzymatic solution (1.0 U mL(-1) ), 1-naphtyl acetate substrate (1.5 mg mL(-1) ) and Fast Blue B salt (1.0 mg mL(-1) ). TLC-MS interface was used to directly elute the active zones into a mass spectrometer or to a micro-vial for further off-line studies. RESULTS: Two AChE inhibitory bands were detected in peel extracts. An analysis via HPTLC-MS and high-performance liquid chromatography diode array detector tandem mass spectrometry (HPLC-DAD-MS/MS) allowed to characterise three potential AChE inhibitors: anonaine (m/z 266 [M + H](+) ; UV lambda(max) = 269.6 nm), glaucine (m/z 256 [M + H](+) ; UV lambda(max) = 282.9 and 300.6 nm) and Xylopine (m/z 296 [M + H](+) ; UV lambda(max) = 278.5 nm). CONCLUSIONS: The application of this optimised high throughput method allowed to establish the presence of three potential AChE inhibitors in cherimoya peel. For the first time AChE inhibitory capacity of these alkaloids is reported.
Xylopine Induces Oxidative Stress and Causes G(2)/M Phase Arrest, Triggering Caspase-Mediated Apoptosis by p53-Independent Pathway in HCT116 Cells.[Pubmed:29362667]
Oxid Med Cell Longev. 2017;2017:7126872.
Xylopine is an aporphine alkaloid that has cytotoxic activity to cancer cells. In this study, the underlying mechanism of Xylopine cytotoxicity was assessed in human colon carcinoma HCT116 cells. Xylopine displayed potent cytotoxicity in different cancer cell lines in monolayer cultures and in a 3D model of cancer multicellular spheroids formed from HCT116 cells. Typical morphology of apoptosis, cell cycle arrest in the G(2)/M phase, increased internucleosomal DNA fragmentation, loss of the mitochondrial transmembrane potential, and increased phosphatidylserine externalization and caspase-3 activation were observed in Xylopine-treated HCT116 cells. Moreover, pretreatment with a caspase-3 inhibitor (Z-DEVD-FMK), but not with a p53 inhibitor (cyclic pifithrin-alpha), reduced Xylopine-induced apoptosis, indicating induction of caspase-mediated apoptosis by the p53-independent pathway. Treatment with Xylopine also caused an increase in the production of reactive oxygen/nitrogen species (ROS/RNS), including hydrogen peroxide and nitric oxide, but not superoxide anion, and reduced glutathione levels were decreased in Xylopine-treated HCT116 cells. Application of the antioxidant N-acetylcysteine reduced the ROS levels and Xylopine-induced apoptosis, indicating activation of ROS-mediated apoptosis pathway. In conclusion, Xylopine has potent cytotoxicity to different cancer cell lines and is able to induce oxidative stress and G(2)/M phase arrest, triggering caspase-mediated apoptosis by the p53-independent pathway in HCT116 cells.
[Studies on alkaloids from Fissistigma oldhamii].[Pubmed:28914026]
Zhongguo Zhong Yao Za Zhi. 2016 Aug;41(15):2838-2842.
14 alkaloids were obtained from stems and leaves of Fissistigma oldhamii, by silica gel, ODS, Sephadex LH-20 column chromatographies, and semi-preparative HPLC. Using physicochemical and spectral methods, the isolated alkaloids were identified as norcepharadione B(1), asimilobine(2), lanuginosine(3), laurotanine(4), isocorydine(5), anolobine(6), Xylopine(7), N-methylbuxifoline(8), aristolactam AIIIa(9), piperumbellactam A(10), goniopedaline(11), aristololactam BIII(12), liriodenine(13), and salutaridine(14), respectively. Compounds 3-5, 8, 10, 11 and 14 were isolated from the genus Fissistigma for the first time.
Revealing the potency of Annona muricata leaves extract as FOXO1 inhibitor for diabetes mellitus treatment through computational study.[Pubmed:28653156]
In Silico Pharmacol. 2016 Dec;5(1):3.
FOXO1 protein inactivation in the nucleus is one of targets for the treatment of diabetes mellitus. Annona muricata leaves contain flavonoid and phenolic compound alkaloids that were known to be able to increase pancreatic beta cell proliferation in animal experiment. This research aimed to predict the active compound ability of the Annona muricata leaves to bind and inhibit FOXO1 protein through in silico study. Analysis of molecular docking was performed by using Autodock Vina PyRx. this research proved that anonaine, rutin, muricatocin a, isolaureline, Xylopine, and kaempferol 3-O-rutinoside had an equal or smaller free binding energy compared to the control compound. Rutin and Muricatocin A had the same binding ability toward 66% amino acid residues, compared to control compound with hydrogen bond type, while Xylopine, anonaine, isolaureline, kaempferol 3-O-rutinoside had a similar binding ability towards 33% amino acid residues compared to control compound with hydrogen bond type.
Cytotoxic Alkaloids from the Stem of Xylopia laevigata.[Pubmed:27399666]
Molecules. 2016 Jul 8;21(7):890.
Xylopia laevigata (Annonaceae), known locally as "meiu" or "pindaiba", is widely used in folk medicine in Northeastern Brazil. In the present work, we performed phytochemical analyses of the stem of X. laevigata, which led to the isolation of 19 alkaloids: (-)-roemerine, (+)-anonaine, lanuginosine, (+)-glaucine, (+)-Xylopine, oxoglaucine, (+)-norglaucine, asimilobine, (-)-xylopinine, (+)-norpurpureine, (+)-N-methyllaurotetanine, (+)-norpredicentrine, (+)-discretine, (+)-calycinine, (+)-laurotetanine, (+)-reticuline, (-)-corytenchine, (+)-discretamine and (+)-flavinantine. The in vitro cytotoxic activity toward the tumor cell lines B16-F10 (mouse melanoma), HepG2 (human hepatocellular carcinoma), K562 (human chronic myelocytic leukemia) and HL-60 (human promyelocytic leukemia) and non-tumor peripheral blood mononuclear cells (PBMCs) was tested using the Alamar Blue assay. Lanuginosine, (+)-Xylopine and (+)-norglaucine had the highest cytotoxic activity. Additionally, the pro-apoptotic effects of lanuginosine and (+)-Xylopine were investigated in HepG2 cells using light and fluorescence microscopies and flow cytometry-based assays. Cell morphology consistent with apoptosis and a marked phosphatidylserine externalization were observed in lanuginosine- and (+)-Xylopine-treated cells, suggesting induction of apoptotic cell death. In addition, (+)-Xylopine treatment caused G(2)/M cell cycle arrest in HepG2 cells. These data suggest that X. laevigata is a potential source for cytotoxic alkaloids.
Anti cancer activity on Graviola, an exciting medicinal plant extract vs various cancer cell lines and a detailed computational study on its potent anti-cancerous leads.[Pubmed:23889049]
Curr Top Med Chem. 2013;13(14):1666-73.
Nature is the world's best chemist: Many naturally occurring compounds have very complicated structures that present great challenges to chemists wishing to determine their structures or replicate them. The plant derived herbal compounds have a long history of clinical use, better patient tolerance and acceptance. Their high ligand binding affinity to the target introduce the prospect of their use in chemo preventive applications; in addition they are freely available natural compounds that can be safely used to prevent various ailments. Plants became the basis of traditional medicine system throughout the world for thousands of years and continue to provide mankind with new remedies. Here, we present a research study on a medicinal plant, Graviola, a native of North America but rarely grown in India. It has a wide potent anticancerous agents coined as Acetogenins which play a key role towards many varieties of cancer, Acetogenins are potent inhibitors of NADH oxidase of the plasma membranes of cancer cells. Potent leads were taken for the study through literature survey, major types of cancer targets were identified, the natureceuticals and the cancer protein were subjected to docking analysis, further with the help of the dock score and other descriptor properties top ranked molecules were collected, commercial drug was also selected and identified as a Test compound for the study. Later, the phytochemicals were subjected to toxicity analysis. Those screened compounds were then considered for active site analysis and to find the best binding site for the study. R Programming library was used to find the best leads. Phytochemicals such as Anonaine, Friedelin, Isolaureline, Annonamine, Anomurine, Kaempferol, Asimilobine, Quercetin, Xylopine were clustered and the highly clustered compounds such as Annonamine , Kaempferol termed to be a potential lead for the study. Further study on experimental analysis may prove the potentiality of these compounds. In the experimental analysis, Graviola leaves were collected, and the extracted components were tested against the HeLa cell line and PC3 cell line. HeLa cells treated with 75 mug of a crude leaf extract of A. muricata showing 80% of cell inhibition. Further investigation of other experimental studies may confirm that these potential lead could make a great impact in which it could help to accelerate the pipeline of drug discovery.
Two birds with one stone? Possible dual-targeting H1N1 inhibitors from traditional Chinese medicine.[Pubmed:22215997]
PLoS Comput Biol. 2011 Dec;7(12):e1002315.
The H1N1 influenza pandemic of 2009 has claimed over 18,000 lives. During this pandemic, development of drug resistance further complicated efforts to control and treat the widespread illness. This research utilizes traditional Chinese medicine Database@Taiwan (TCM Database@Taiwan) to screen for compounds that simultaneously target H1 and N1 to overcome current difficulties with virus mutations. The top three candidates were de novo derivatives of Xylopine and rosmaricine. Bioactivity of the de novo derivatives against N1 were validated by multiple machine learning prediction models. Ability of the de novo compounds to maintain CoMFA/CoMSIA contour and form key interactions implied bioactivity within H1 as well. Addition of a pyridinium fragment was critical to form stable interactions in H1 and N1 as supported by molecular dynamics (MD) simulation. Results from MD, hydrophobic interactions, and torsion angles are consistent and support the findings of docking. Multiple anchors and lack of binding to residues prone to mutation suggest that the TCM de novo derivatives may be resistant to drug resistance and are advantageous over conventional H1N1 treatments such as oseltamivir. These results suggest that the TCM de novo derivatives may be suitable candidates of dual-targeting drugs for influenza.


