AjugoseCAS# 512-72-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 512-72-1 | SDF | Download SDF |
| PubChem ID | 441421.0 | Appearance | Powder |
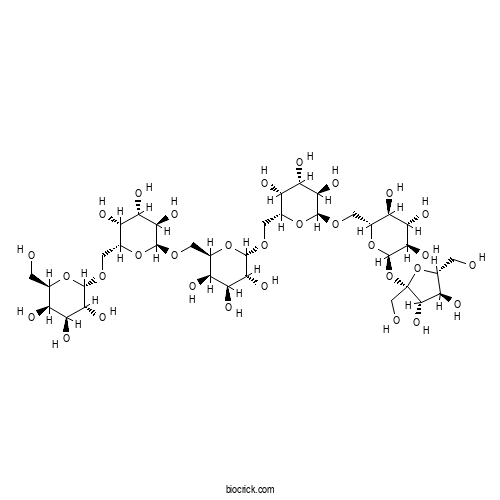
| Formula | C36H62O31 | M.Wt | 990.86 |
| Type of Compound | Oligoses | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2S,3R,4S,5R,6R)-2-[[(2R,3R,4S,5R,6S)-6-[[(2R,3R,4S,5R,6S)-6-[[(2R,3R,4S,5R,6S)-6-[[(2R,3S,4S,5R,6R)-6-[(2S,3S,4S,5R)-3,4-dihydroxy-2,5-bis(hydroxymethyl)oxolan-2-yl]oxy-3,4,5-trihydroxyoxan-2-yl]methoxy]-3,4,5-trihydroxyoxan-2-yl]methoxy]-3,4,5-trihydroxyoxan-2-yl]methoxy]-3,4,5-trihydroxyoxan-2-yl]methoxy]-6-(hydroxymethyl)oxane-3,4,5-triol | ||
| SMILES | C(C1C(C(C(C(O1)OCC2C(C(C(C(O2)OCC3C(C(C(C(O3)OCC4C(C(C(C(O4)OCC5C(C(C(C(O5)OC6(C(C(C(O6)CO)O)O)CO)O)O)O)O)O)O)O)O)O)O)O)O)O)O)O)O | ||
| Standard InChIKey | NCIHJQNXRKJRMJ-HKJJPIHFSA-N | ||
| Standard InChI | InChI=1S/C36H62O31/c37-1-8-14(40)20(46)25(51)31(61-8)57-3-10-15(41)21(47)26(52)32(62-10)58-4-11-16(42)22(48)27(53)33(63-11)59-5-12-17(43)23(49)28(54)34(64-12)60-6-13-18(44)24(50)29(55)35(65-13)67-36(7-39)30(56)19(45)9(2-38)66-36/h8-35,37-56H,1-7H2/t8-,9-,10-,11-,12-,13-,14+,15+,16+,17+,18-,19-,20+,21+,22+,23+,24+,25-,26-,27-,28-,29-,30+,31+,32+,33+,34+,35-,36+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Ajugose Dilution Calculator

Ajugose Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.0092 mL | 5.0461 mL | 10.0922 mL | 20.1845 mL | 25.2306 mL |
| 5 mM | 0.2018 mL | 1.0092 mL | 2.0184 mL | 4.0369 mL | 5.0461 mL |
| 10 mM | 0.1009 mL | 0.5046 mL | 1.0092 mL | 2.0184 mL | 2.5231 mL |
| 50 mM | 0.0202 mL | 0.1009 mL | 0.2018 mL | 0.4037 mL | 0.5046 mL |
| 100 mM | 0.0101 mL | 0.0505 mL | 0.1009 mL | 0.2018 mL | 0.2523 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Dihydrocatalpol
Catalog No.:BCX1287
CAS No.:6736-86-3
- Isobellidifolin
Catalog No.:BCX1286
CAS No.:552-00-1
- Napelline
Catalog No.:BCX1285
CAS No.:5008-52-6
- Morusignin L
Catalog No.:BCX1284
CAS No.:149733-95-9
- 5-Hydroxy-3,7-dimethoxyflavone
Catalog No.:BCX1283
CAS No.:70786-48-0
- 1-(2,6-Dimethoxyphenyl)-3-(4-hydroxyphenyl)-2-propen-1-one
Catalog No.:BCX1282
CAS No.:85679-87-4
- Ethyl rosmarinate
Catalog No.:BCX1281
CAS No.:174591-47-0
- 3,5,7-Trimethoxyflavone
Catalog No.:BCX1280
CAS No.:26964-29-4
- Micromarin F
Catalog No.:BCX1279
CAS No.:73292-93-0
- Palvanil
Catalog No.:BCX1278
CAS No.:69693-13-6
- N-Acetylcytisine
Catalog No.:BCX1277
CAS No.:6018-52-6
- Hispidol
Catalog No.:BCX1276
CAS No.:5786-54-9
- Limocitrin 3-O-β-D-glucopyranoside
Catalog No.:BCX1289
CAS No.:38836-51-0
- Wistin
Catalog No.:BCX1290
CAS No.:19046-26-5
- Physcion-8-O-β-gentiobioside
Catalog No.:BCX1291
CAS No.:84268-38-2
- Chaparrinone
Catalog No.:BCX1292
CAS No.:22611-34-3
- Ajugasterone C 2-acetate
Catalog No.:BCX1293
CAS No.:154510-93-7
- t-OMe-Byakangelicin
Catalog No.:BCX1294
CAS No.:79638-04-3
- Amaroswerin
Catalog No.:BCX1295
CAS No.:21233-18-1
- Hypoglaunine D
Catalog No.:BCX1296
CAS No.:220751-00-8
- Kanshone C
Catalog No.:BCX1297
CAS No.:117634-64-7
- Lyciumin A
Catalog No.:BCX1298
CAS No.:125708-06-7
- Xylopine
Catalog No.:BCX1299
CAS No.:517-71-5
- 16α-hydroxy-3-oxo-lanosta-7,9(11),24-trien-21-oic acid
Catalog No.:BCX1300
CAS No.:862109-64-6
HPLC profiling of flatulence and non-flatulence saccharides in eleven ricebean (Vigna umbellata) varieties from North-East India.[Pubmed:30956347]
J Food Sci Technol. 2019 Mar;56(3):1655-1662.
HPLC method was optimized for analysis eight flatulence and non-flatulence saccharides in 11 ricebean varieties using Spherisorb NH(2) chromatographic column within 20 min by isocratic mobile phase containing acetonitrile and water in ratio of 70:30 at flow rate of 1 mL/min. The glucose, sucrose, raffinose, stachyose, verbascose and Ajugose content in these varieties ranged from 157.63-365.08, 202.61-997.74, 196.29-429.5, 692.7-1480.67, 47.66-130.36 and 0.27-8.82 mg/100 g, respectively. The total flatulence saccharides (FS) were range between 937.13 +/- 44.31 (JCR-08-12) and 1944.55 +/- 83.57 (JCR-08-32) mg/100 g. Among the total FS, the content of stachyose was found highest in all varieties than other FS. Principal component analysis (PCA) revealed that glucose, sucrose, raffinose, stachyose, verbascose, Ajugose, and total FS contributed 50.03% variation among the varieties. The eleven varieties were classified into three groups based on saccharides content by PCA. The group-A (JCR-08-12) and group-B (JCR-08-7, JCR-08-16, JCR-50, JCR-13-11, JCR-13-13 and Nagadal) had very low and low content of total FS, respectively.
Significance of galactinol and raffinose family oligosaccharide synthesis in plants.[Pubmed:26379684]
Front Plant Sci. 2015 Aug 26;6:656.
Abiotic stress induces differential expression of genes responsible for the synthesis of raffinose family of oligosaccharides (RFOs) in plants. RFOs are described as the most widespread D-galactose containing oligosaccharides in higher plants. Biosynthesis of RFOs begin with the activity of galactinol synthase (GolS; EC 2.4.1.123), a GT8 family glycosyltransferase that galactosylates myo-inositol to produce galactinol. Raffinose and the subsequent higher molecular weight RFOs (Stachyose, Verbascose, and Ajugose) are synthesized from sucrose by the subsequent addition of activated galactose moieties donated by Galactinol. Interestingly, GolS, the key enzyme of this pathway is functional only in the flowering plants. It is thus assumed that RFO synthesis is a specialized metabolic event in higher plants; although it is not known whether lower plant groups synthesize any galactinol or RFOs. In higher plants, several functional importance of RFOs have been reported, e.g., RFOs protect the embryo from maturation associated desiccation, are predominant transport carbohydrates in some plant families, act as signaling molecule following pathogen attack and wounding and accumulate in vegetative tissues in response to a range of abiotic stresses. However, the loss-of-function mutants reported so far fail to show any perturbation in those biological functions. The role of RFOs in biotic and abiotic stress is therefore still in debate and their specificity and related components remains to be demonstrated. The present review discusses the biology and stress-linked regulation of this less studied extension of inositol metabolic pathway.
Hydrophilic interaction liquid chromatography for the separation, purification, and quantification of raffinose family oligosaccharides from Lycopus lucidus Turcz.[Pubmed:26011699]
J Sep Sci. 2015 Aug;38(15):2607-13.
A systematic strategy based on hydrophilic interaction liquid chromatography was developed for the separation, purification and quantification of raffinose family oligosaccharides from Lycopus lucidus Turcz. Methods with enough hydrophilicity and selectivity were utilized to resolve the problems encountered in the separation of oligosaccharides such as low retention, low resolution and poor solubility. The raffinose family oligosaccharides in L. lucidus Turcz. were isolated using solid-phase extraction followed by hydrophilic interaction liquid chromatography at semi-preparative scale to obtain standards of stachyose, verbascose and Ajugose. Utilizing the obtained oligosaccharides as standards, a quantitative determination method was developed, validated and applied for the content determination of raffinose family oligosaccharides both in the aerial and root parts of L. lucidus Turcz. There were no oligosaccharides in the aerial parts, while in the root parts, the total content was 686.5 mg/g with the average distribution: raffinose 66.5 mg/g, stachyose 289.0 mg/g, verbascose 212.4 mg/g, and Ajugose 118.6 mg/g. The result provided the potential of roots of L. lucidus Turcz. as new raffinose family oligosaccharides sources for functional food. Moreover, since the present systematic strategy is efficient, sensitive and robust, separation, purification and quantification of oligosaccharides by hydrophilic interaction liquid chromatography seems to be possible.
Isolation and structural analysis of ajugose from Vigna mungo L.[Pubmed:16716272]
Carbohydr Res. 2006 Sep 4;341(12):2156-60.
The hexasaccharide Ajugose, alpha-D-galactopyranosyl-(1-->6)-alpha-D-galactopyranosyl-(1-->6)-O-alpha-D-galactopyranosyl-(1-->6)-alpha-D-galactopyranosyl-(1-->6)-alpha-D-glucopyranosyl-(1<-->2)-beta-D-fructofuranoside, generally uncommon in legumes, was detected in the seeds of Vigna mungo L. by TLC and paper chromatography. Ajugose was then isolated by silica gel chromatography and its structure was established by acid and enzymatic hydrolysis, fast atom bombardment mass spectrometry and both one- and two-dimensional 1H and 13C NMR techniques.
Oligosaccharins of black gram (Vigna mungo L.) as affected by processing methods.[Pubmed:16395628]
Plant Foods Hum Nutr. 2005 Dec;60(4):173-80.
The oligosaccharide content was determined in 12 different cultivars of black gram. The effect of various treatments such as soaking, cooking, and enzyme treatment on the raffinose family oligosaccharides of dry seeds and flour was studied. Ajugose, a higher oligosaccharide (DP 6) found in trace quantities in seeds, was shown in black gram by HPLC. The percent reduction of raffinose, stachyose, verbascose, and Ajugose after soaking for 16 hr was 41.66%, 47.61%, 28.48%, and 26.82%, respectively in Local-I variety and 43.75%, 20.58%, 23.60%, and 15.88%, respectively in Local-II variety. Cooking for 60 min resulted in decrease of 100% for raffinose, 76.19% for stachyose, 36.39% for verbascose, and 60.97% for Ajugose in Local-I variety and 100% for raffinose, 55.88% for stachyose, 48.52% for verbascose, and 56.07% for Ajugose in Local-II variety. Thin layer chromatographic analysis of 3 hr enzyme-treated samples revealed almost complete hydrolysis of raffinose family of oligosaccharides. Among the different methods employed, enzyme treatment was found to be the most effective for removing alpha-galactosides in black gram.
Compositional variations for alpha-galactosides in different species of leguminosae, brassicaceae, and barley: a chemotaxonomic study based on chemometrics and high-performance capillary electrophoresis.[Pubmed:15998152]
J Agric Food Chem. 2005 Jul 13;53(14):5809-17.
The contents of raffinose family oligosaccharides (RFO) and sucrose in Brassica, Lupinus, Pisum, and Hordeum species were investigated by chemometric principal component analysis (PCA). Hordeum samples contained sucrose and raffinose, and Brassica samples all contained sucrose, raffinose, and stachyose. In addition to these, the Pisum samples contained verbascose and the Lupinus samples also contained Ajugose. High stachyose and low Ajugose contents were found in Lupinus albus in contrast to Lupinus angustifolius, having low stachyose and high Ajugose contents. Lupinus luteus had average stachyose and Ajugose contents, whereas large amounts of verbascose were accumulated in these seeds. Lupinus mutabilis had high stachyose and low Ajugose contents, similar to the composition in L. albus but showing higher raffinose content. The Brassica samples also showed compositional RFO variations within the species, and subgroup formations were discovered within the investigated Brassica napus varieties. PCA results indicated compositional variations between the investigated genera and within the various species of value as chemotaxonomic defined parameters and as tools in evaluations of authenticity/falsifications when RFO-containing plants are used as, for example, feed and food additives.
High-performance capillary electrophoresis with indirect UV detection for determination of alpha-galactosides in Leguminosae and Brassicaceae.[Pubmed:14558752]
J Agric Food Chem. 2003 Oct 22;51(22):6391-7.
A rapid, easy, and reproducible capillary electrophoresis method for determination of raffinose family oligosaccharides (alpha-galactosides) was developed. Sucrose, raffinose, stachyose, verbascose, and Ajugose were determined with indirect UV detection at moderate alkaline pH 9.2, using pyridine-2,6-dicarboxylic acid as background electrolyte in a sodium tetraborate buffer with added cetyltrimethylammonium bromide. The separation efficiency measured by the number of theoretical plates (N) ranged from 1.4 x 10(5) to 2.3 x 10(5). The precision of the method, measured by the relative standard deviation (RSD), was less than 0.53% for the migration times and better than 3.4% for normalized areas (NA), considering all sugars except verbascose (RSD(NA) = 11.8%). Detection limits were about 110 microg/mL, corresponding to 150-320 microM. Relative response factors (RRF) were calculated on the basis of linearity studies and used for quantification of alpha-galactosides in a lupine sample (Lupinus angustifolius).
Investigations into genotypic variations of peanut carbohydrates.[Pubmed:10725144]
J Agric Food Chem. 2000 Mar;48(3):750-6.
Carbohydrates are known to be important precursors in the development of roasted peanut quality. However, little is known about their genotypic variation. A better understanding of the role of carbohydrates in roasted peanut quality requires first an understanding of the genotypic variation in the soluble carbohydrate components. Ion exchange chromatography was used to isolate 20 different carbohydrate components in 52 genotypes grown in replicated trials at two locations. Inositol, glucose, fructose, sucrose, raffinose, and stachyose were quantitated, and 12 unknown peaks were evaluated on the basis of the peak height of the unknown relative to the cellobiose internal standard peak height. Peaks tentatively identified as verbascose and Ajugose could not be properly integrated because of tailing. Of the 18 carbohydrates that were estimated, 9 exhibited significant variation between test environments, 5 among market types, 14 among genotypes within market types, and 11 exhibited some significant form of genotype x environment interaction. Genotypes accounted for 38-78% of the total variation for the known components, suggesting that broad-sense heritability for these components is high. The observed high genotypic variation in carbohydrate components is similar to the high genotypic variation observed for the sweetness attribute in roasted peanuts, which raises the question regarding possible interrelationships. The establishment of such interrelationships could be most beneficial to peanut breeding programs to ensure the maintenance of flavor quality in future peanut varieties.
[Studies on chemical constituents of the roots of Lantana camara].[Pubmed:1442083]
Yao Xue Xue Bao. 1992;27(7):515-21.
Six oligosaccharides (I-VI) and six iridoid glucosides (VII-XII) isolated from the ethanolic extract of Lantana camara roots were identified as stachyose (I), verbascose (II), Ajugose (III), verbascotetracose (IV), alpha-D-galac-(1-[-6)-alpha-D-galac(-1](3)-6-D-gluc(V ) , alpha-D-galac-(1-6)-alpha-D-galac(-1]-(4)6-D-)gluc(VI) , theveside (VII), 8-epiloganin (VIII), shanzhsid methyl ester (IX), theviridoside (X), lamiridoside (XI) and geniposide (XII), on the basis of spectral analysis (1H-NMR, 13C-NMR, FD-MS, GC-MS), physico-chemical constants and preparation of derivatives. V and VI were new compounds named lantanose A and lantanose B, respectively. The others were isolated from this plant for the first time.
Characterization and distribution of soluble and insoluble carbohydrates in lupin seeds.[Pubmed:7445755]
Z Lebensm Unters Forsch. 1980 Oct;171(4):281-5.
White, blue and yellow lupin seeds were analyzed for their soluble and insoluble carbohydrate contents. The seeds contained only traces of starch. Their furfural generator contents were fairly constant (9.3--10.5%) and their soluble sugar contents were in the range of 11.8 to 14.1%. Thin-layer and column chromatography of the ethanol-soluble sugars showed the presence of varying amounts of Ajugose, verbascose, stachyose, raffinose and sucrose. Quantitative analysis revealed an average of 25% sucrose in the total sugars, the major part of which is composed of alpha-galactosides. Separate analysis of the cotyledons (including the germ) showed that the latter contain 80% total carbohydrates, most of which were structural polysaccharides. The composition of the cell-wall constituents was examined after acid hydrolysis. Monosaccharides resulting after acid hydrolysis of the hemicelluloses were predominantly xylose and arabinose. Smaller quantities of galactose and glucose, and traces of rhamnose were also present. The cotyledons contain a considerable amount of soluble sugars (19.0%). Small amounts of glucose-containing polymers, soluble in water and dilute acid, were present in the cotyledons (1.0%); they increased to 2.4% in the hulls.


