5-Hydroxy-3,7-dimethoxyflavoneCAS# 70786-48-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 70786-48-0 | SDF | Download SDF |
| PubChem ID | 5748697.0 | Appearance | Powder |
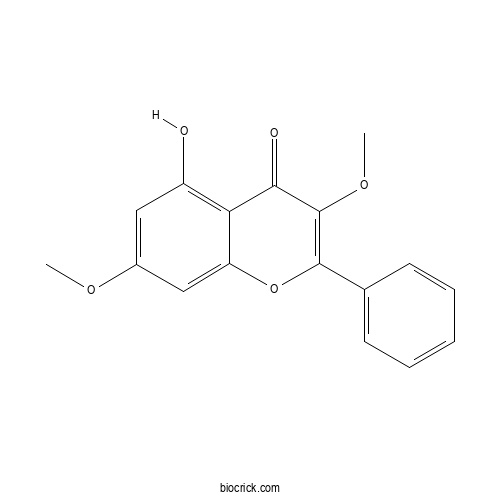
| Formula | C17H14O5 | M.Wt | 298.29 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 5-hydroxy-3,7-dimethoxy-2-phenylchromen-4-one | ||
| SMILES | COC1=CC(=C2C(=C1)OC(=C(C2=O)OC)C3=CC=CC=C3)O | ||
| Standard InChIKey | OYCOUDKDRFJOCP-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C17H14O5/c1-20-11-8-12(18)14-13(9-11)22-16(17(21-2)15(14)19)10-6-4-3-5-7-10/h3-9,18H,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

5-Hydroxy-3,7-dimethoxyflavone Dilution Calculator

5-Hydroxy-3,7-dimethoxyflavone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.3524 mL | 16.7622 mL | 33.5244 mL | 67.0488 mL | 83.8111 mL |
| 5 mM | 0.6705 mL | 3.3524 mL | 6.7049 mL | 13.4098 mL | 16.7622 mL |
| 10 mM | 0.3352 mL | 1.6762 mL | 3.3524 mL | 6.7049 mL | 8.3811 mL |
| 50 mM | 0.067 mL | 0.3352 mL | 0.6705 mL | 1.341 mL | 1.6762 mL |
| 100 mM | 0.0335 mL | 0.1676 mL | 0.3352 mL | 0.6705 mL | 0.8381 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 1-(2,6-Dimethoxyphenyl)-3-(4-hydroxyphenyl)-2-propen-1-one
Catalog No.:BCX1282
CAS No.:85679-87-4
- Ethyl rosmarinate
Catalog No.:BCX1281
CAS No.:174591-47-0
- 3,5,7-Trimethoxyflavone
Catalog No.:BCX1280
CAS No.:26964-29-4
- Micromarin F
Catalog No.:BCX1279
CAS No.:73292-93-0
- Palvanil
Catalog No.:BCX1278
CAS No.:69693-13-6
- N-Acetylcytisine
Catalog No.:BCX1277
CAS No.:6018-52-6
- Hispidol
Catalog No.:BCX1276
CAS No.:5786-54-9
- 2',4,4',6'-Tetramethoxychalcone
Catalog No.:BCX1275
CAS No.:94103-36-3
- 2'-O-Methylphloretin
Catalog No.:BCX1274
CAS No.:111316-17-7
- Phenoxodiol
Catalog No.:BCX1273
CAS No.:81267-65-4
- 6-Hydroxyluteolin
Catalog No.:BCX1272
CAS No.:18003-33-3
- Sinomenine N-oxide
Catalog No.:BCX1271
CAS No.:1000026-77-6
- Morusignin L
Catalog No.:BCX1284
CAS No.:149733-95-9
- Napelline
Catalog No.:BCX1285
CAS No.:5008-52-6
- Isobellidifolin
Catalog No.:BCX1286
CAS No.:552-00-1
- Dihydrocatalpol
Catalog No.:BCX1287
CAS No.:6736-86-3
- Ajugose
Catalog No.:BCX1288
CAS No.:512-72-1
- Limocitrin 3-O-β-D-glucopyranoside
Catalog No.:BCX1289
CAS No.:38836-51-0
- Wistin
Catalog No.:BCX1290
CAS No.:19046-26-5
- Physcion-8-O-β-gentiobioside
Catalog No.:BCX1291
CAS No.:84268-38-2
- Chaparrinone
Catalog No.:BCX1292
CAS No.:22611-34-3
- Ajugasterone C 2-acetate
Catalog No.:BCX1293
CAS No.:154510-93-7
- t-OMe-Byakangelicin
Catalog No.:BCX1294
CAS No.:79638-04-3
- Amaroswerin
Catalog No.:BCX1295
CAS No.:21233-18-1
Flavones isolated from Pseudognaphalium liebmannii with tracheal smooth muscle relaxant properties.[Pubmed:38189356]
Nat Prod Res. 2024 Jan 8:1-6.
The inflorescences of Pseudognaphalium liebmannii are used as folk medicine to treat various respiratory diseases. In this work, we report the isolation of seven known flavones: 5-Hydroxy-3,7-dimethoxyflavone 1, 5,8-dihydroxy-3,7-dimethoxyflavone 2, 5,7-dihydroxy-3,8-dimethoxyflavone 3 (gnaphaliin A), 3,5-dihydroxy-7,8-dimethoxyflavone 4 (gnaphaliin B), 3,5-dihydroxy-6,7,8-trimethoxyflavone 5, 3,5,7-trimethoxyflavone 6 and 3-O-methylquercetin 7. All these flavones except 1 and 6 showed a relaxant effect on guinea pig tracheal preparation with EC(50) between 69.91 +/- 15.32 and 118.72 +/- 7.06 microM. Aminophylline (EC50 = 122.03 +/- 7.05 microM) was used as a relaxant reference drug. The active flavones shifted the concentration-response curves of forskolin and nitroprusside leftward, and significantly reduced the EC(50) values of these drugs. Furthermore, these flavones dose-dependently inhibited phosphodiesterase (PDE) in an in vitro assay. This reveals that the inflorescences of P. liebmannii contain several flavones with relaxant effect on airway smooth muscle and with PDEs inhibition that contribute to supporting the anti-asthmatic traditional use.
Demethylation of Polymethoxyflavones by Human Gut Bacterium, Blautia sp. MRG-PMF1.[Pubmed:28211698]
J Agric Food Chem. 2017 Mar 1;65(8):1620-1629.
Polymethoxyflavones (PMFs) were biotransformed to various demethylated metabolites in the human intestine by the PMF-metabolizing bacterium, Blautia sp. MRG-PMF1. Because the newly formed metabolites can have different biological activities, the pathways and regioselectivity of PMF bioconversion were investigated. Using an anaerobic in vitro study, 12 PMFs, 5,7-dimethoxyflavone (5,7-DMF), 5-hydroxy-7-methoxyflavone (5-OH-7-MF), 3,5,7-trimethoxyflavone (3,5,7-TMF), 5-Hydroxy-3,7-dimethoxyflavone (5-OH-3,7-DMF), 5,7,4'-trimethoxyflavone (5,7,4'-TMF), 5-hydroxy-7,4'-dimethoxyflavone (5-OH-7,4'-DMF), 3,5,7,4'-tetramethoxyflavone (3,5,7,4'-TMF), 5-hydroxy-3,7,4'-trimethoxyflavone (5-OH-3,7,4'-TMF), 5,7,3',4'-tetramethoxyflavone (5,7,3',4'-TMF), 3,5,7,3',4'-pentamethoxyflavone (3,5,7,3',4'-PMF), 5-hydroxy-3,7,3',4'-tetramethoxyflavone (5-OH-3,7,3',4'-TMF), and 5,3'-dihydroxy-3,7,4'-trimethoxyflavone (5,3'-diOH-3,7,4'-TMF), were converted to chrysin, apigenin, galangin, kaempferol, luteolin, and quercetin after complete demethylation. The time-course monitoring of PMF biotransformations elucidated bioconversion pathways, including the identification of metabolic intermediates. As a robust flavonoid demethylase, regioselectivity of PMF demethylation generally followed the order C-7 > C-4' approximately C-3' > C-5 > C-3. PMF demethylase in the MRG-PMF1 strain was suggested as a Co-corrinoid methyltransferase system, and this was supported by the experiments utilizing other methyl aryl ether substrates and inhibitors.
Antimicrobial activity of Calotropis procera Ait. (Asclepiadaceae) and isolation of four flavonoid glycosides as the active constituents.[Pubmed:23417281]
World J Microbiol Biotechnol. 2013 Jul;29(7):1255-62.
Antimicrobial activity of solvent extracts and flavonoids of Calotropis procera growing wild in Saudi Arabia was evaluated using the agar well-diffusion method. A bioassay-guided fractionation of the crude flavonoid fraction (Cf3) of MeOH extract which showed the highest antimicrobial activity led to the isolation of four flavonoid glycosides as the bioactive constituents. Structure of compounds have been elucidated using physical and spectroscopic methods including (UV, IR, (1)H, (13)C-NMR, DEPT, 2D (1)H-(1)H COSY, HSQC, HMBC and NOESY). Compounds were found to be the 3-O-rutinosides of quercetin, kaempferol and isorhamnetin, besides the flavonoid 5-Hydroxy-3,7-dimethoxyflavone-4'-O-beta-glucopyranoside. Most of the isolated extracts showed antimicrobial activity against the test microorganisms, where the crude flavonoid fraction was the most active, diameter of inhibition zones ranged between 15.5 and 28.5 mm against the tested bacterial strains, while reached 30 mm against the fungal Candida albicans. The minimal inhibitory concentrations varied from 0.04 to 0.32 mg/ml against all of the tested microorganisms in case of the crude flavonoid fraction. Quercetin-3-O-rutinoside showed superior activity over the remainder flavonoids. The Gram-positive bacteria (Staphylococcus aureus and Bacillus subtilis) were more susceptible than the Gram-negative (Pseudomonas aeruginosa and Salmonella enteritidis) and the yeast species were more susceptible than the filamentous fungi. The study recommend the use of such natural products as antimicrobial biorationals.
Isolation and identification of antibacterial and cytotoxic compounds from the leaves of Muntingia calabura L.[Pubmed:23276785]
J Ethnopharmacol. 2013 Mar 7;146(1):198-204.
ETHNOPHARMACOLOGICAL RELEVANCE: Muntingia calabura (Elaeocarpaceae) is one of the most common roadside trees in Malaysia. Its leaves, barks, flowers and roots have been used as a folk remedy for the treatment of fever, incipient cold, liver disease, as well as an antiseptic agent in Southeast Asia. The aim of this study is to isolate and identify the antibacterial and cytotoxic compounds from the leaves of Muntingia calabura L. MATERIALS AND METHODS: Antibacterial and cytotoxic activities were determined by micro-broth dilution and MTT assays, respectively. Seven fractions (F1-F7), three flavones and a chalcone were isolated from the active EtOAc extract using bioassay-guided screening. The structures of four compounds were elucidated by spectroscopic methods and compared with published data. The compounds were further tested for their antibacterial and cytotoxic activities. RESULTS: Three flavones and a chalcone [5,7-dihydroxy-3,8-dimethoxyflavone (1), 2',4'-dihydroxychalcone (2), 5-Hydroxy-3,7-dimethoxyflavone (3) and 3,5,7-trihydroxy-8-methoxyflavone (4)] were isolated from the active fraction F5 of EtOAc extract. Compounds 1 and 3 were isolated for the first time from Muntingia calabura L. Antibacterial activity indicates that compound 2 exhibited the most significant activity with MIC value of 50 mug/mL and 100 mug/mL against MSSA and MRSA, respectively. Cytotoxic activity indicates that compounds 2 and 3 exhibited very strong activity against HL60 with IC50 values of 3.43 mug/mL and 3.34 mug/mL, respectively. CONCLUSION: The antibacterial activity of the leaves of Muntingia calabura L. is ascribable to the active compound 2 while the cytotoxic activity is ascribable to the active compounds 2 and 3.
Simultaneous identification and quantitation of 11 flavonoid constituents in Kaempferia parviflora by gas chromatography.[Pubmed:17266972]
J Chromatogr A. 2007 Mar 2;1143(1-2):227-33.
Kaempferia parviflora (Krachaidum; KD) is a Thai herb, the rhizomes of which have been used in folk medicine and ritual ceremonies. The increasing use of KD has led to concerns regarding the variation of quality, potency and efficacy of KD products. A gas chromatographic method was developed and validated using 11 flavonoids that had been fully characterized as reference. Limits of detection ranged from a low of 0.1 ppm to a high of 1.0 ppm. The limits of quantitation were a low of 0.5 ppm (5-Hydroxy-3,7-dimethoxyflavone) to a high of 3.0 ppm (5,7,4'-trimethoxyflavone and 5,7,3',4'-tetramethoxyflavone). Precision of intra- and inter-day analyses gave a RSD range of 3.02-8.25 and 2.84-12.37, respectively. The diversity of flavonoid content and their distribution profiles in KD samples from 12 different origins was investigated using the validated method. Total flavonoid content in these samples ranged from 23.86 to 60.98 mg/g. Two of the compounds, 5,7-dimethoxyflavone and 5,7,4'-trimethoxyflavone, emerged as major constituents. Samples contained as much as 21.68 and 9.88 mg/g, respectively. Two distinct patterns of the distribution of the flavonoids, as characterized by the ratio of these two compounds in the KD rhizome samples, were observed. This method is expected to be useful in the quantitative and qualitative analyses of the flavonoid content of KD samples and as a quality control assessment of KD raw materials and products.


