NapellineCAS# 5008-52-6 |
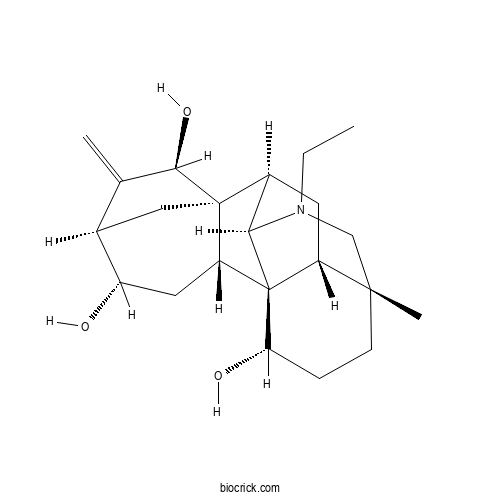
- 12-Epinapelline
Catalog No.:BCN2800
CAS No.:110064-71-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 5008-52-6 | SDF | Download SDF |
| PubChem ID | 165359539.0 | Appearance | Powder |
| Formula | C22H33NO3 | M.Wt | 359.51 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (1S,2R,4S,5R,7R,8R,9R,10R,13R,16S,17R)-11-ethyl-13-methyl-6-methylidene-11-azahexacyclo[7.7.2.15,8.01,10.02,8.013,17]nonadecane-4,7,16-triol | ||
| SMILES | CCN1CC2(CCC(C34C2CC(C31)C56C4CC(C(C5)C(=C)C6O)O)O)C | ||
| Standard InChIKey | AZAZKLKDEOMJBJ-SDQMMUEXSA-N | ||
| Standard InChI | InChI=1S/C22H33NO3/c1-4-23-10-20(3)6-5-17(25)22-15(20)7-13(18(22)23)21-9-12(11(2)19(21)26)14(24)8-16(21)22/h12-19,24-26H,2,4-10H2,1,3H3/t12-,13+,14+,15-,16-,17+,18-,19-,20+,21+,22-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Napelline Dilution Calculator

Napelline Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7816 mL | 13.9078 mL | 27.8156 mL | 55.6313 mL | 69.5391 mL |
| 5 mM | 0.5563 mL | 2.7816 mL | 5.5631 mL | 11.1263 mL | 13.9078 mL |
| 10 mM | 0.2782 mL | 1.3908 mL | 2.7816 mL | 5.5631 mL | 6.9539 mL |
| 50 mM | 0.0556 mL | 0.2782 mL | 0.5563 mL | 1.1126 mL | 1.3908 mL |
| 100 mM | 0.0278 mL | 0.1391 mL | 0.2782 mL | 0.5563 mL | 0.6954 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Morusignin L
Catalog No.:BCX1284
CAS No.:149733-95-9
- 5-Hydroxy-3,7-dimethoxyflavone
Catalog No.:BCX1283
CAS No.:70786-48-0
- 1-(2,6-Dimethoxyphenyl)-3-(4-hydroxyphenyl)-2-propen-1-one
Catalog No.:BCX1282
CAS No.:85679-87-4
- Ethyl rosmarinate
Catalog No.:BCX1281
CAS No.:174591-47-0
- 3,5,7-Trimethoxyflavone
Catalog No.:BCX1280
CAS No.:26964-29-4
- Micromarin F
Catalog No.:BCX1279
CAS No.:73292-93-0
- Palvanil
Catalog No.:BCX1278
CAS No.:69693-13-6
- N-Acetylcytisine
Catalog No.:BCX1277
CAS No.:6018-52-6
- Hispidol
Catalog No.:BCX1276
CAS No.:5786-54-9
- 2',4,4',6'-Tetramethoxychalcone
Catalog No.:BCX1275
CAS No.:94103-36-3
- 2'-O-Methylphloretin
Catalog No.:BCX1274
CAS No.:111316-17-7
- Phenoxodiol
Catalog No.:BCX1273
CAS No.:81267-65-4
- Isobellidifolin
Catalog No.:BCX1286
CAS No.:552-00-1
- Dihydrocatalpol
Catalog No.:BCX1287
CAS No.:6736-86-3
- Ajugose
Catalog No.:BCX1288
CAS No.:512-72-1
- Limocitrin 3-O-β-D-glucopyranoside
Catalog No.:BCX1289
CAS No.:38836-51-0
- Wistin
Catalog No.:BCX1290
CAS No.:19046-26-5
- Physcion-8-O-β-gentiobioside
Catalog No.:BCX1291
CAS No.:84268-38-2
- Chaparrinone
Catalog No.:BCX1292
CAS No.:22611-34-3
- Ajugasterone C 2-acetate
Catalog No.:BCX1293
CAS No.:154510-93-7
- t-OMe-Byakangelicin
Catalog No.:BCX1294
CAS No.:79638-04-3
- Amaroswerin
Catalog No.:BCX1295
CAS No.:21233-18-1
- Hypoglaunine D
Catalog No.:BCX1296
CAS No.:220751-00-8
- Kanshone C
Catalog No.:BCX1297
CAS No.:117634-64-7
12-Epi-Napelline regulated TGF-beta/BMP signaling pathway mediated by BMSCs paracrine acceleration against osteoarthritis.[Pubmed:36252476]
Int Immunopharmacol. 2022 Dec;113(Pt A):109307.
BACKGROUND: This study is to investigate the role of 12-Epi-Napelline, a new alkaloid isolated from aconitum, in promoting the paracrine of Bone Mesenchymal Stem Cells (BMSCs) and the synergistic therapeutic effects on osteoarthritis. METHOD: We tested the cytotoxicity and optimization of 12-Epi-Napelline, and then simulated the osteoarthritis model in vitro damaging the chondrocytes by lipopolysaccharide (LPS) and RT-qPCR, Western blot and Immunofluorescence were used to detect the inflammatory factor IL-1beta, COX-2, TNF-alpha, MMP-13 and anabolic cytokines of Col-2, BMP-2, TGF-beta1 and Sox9 expression in chondrocytes after 12-Epi-Napelline treatment. Under the treatment of different time, Col-2, BMP-2, TGF-beta1 and Sox9 expression in BMSCs were detected by RT-qPCR, Western blot, and Immunofluorescence. By establishing an osteoarthritis model in vivo, the anti-osteoarthritis effect of 12-Epi-Napelline or BMSCs was evaluated. RESULTS: The results showed the expressions of IL-1beta, COX-2, TNF-alpha, and MMP-13 were down-regulated in chondrocytes after 12-Epi-Napelline treatment, while the expression of Col-2, BMP-2, TGF-beta1 and Sox9 were increased to normal chondrocytes. These increased expression also occurred in BMSCs. BMSCs had the trend of transforming into chondrocytes by regulating TGF-beta signaling pathway under the treatment of 12-Epi-Napelline. CONCLUSION: This study could confirm that 12-Epi-Napelline is not only effective in the treatment of osteoarthritis, but also can induce BMSCs to secrete growth factors that promote chondrocyte repair to help repair the damage caused by osteoarthritis.
[Therapeutic effects of alkaloids in Tibetan medicine Bangna (Aconiti Penduli et Aconiti Flavi Radix) on osteoarthritis rats and mechanisms].[Pubmed:36164879]
Zhongguo Zhong Yao Za Zhi. 2022 Sep;47(17):4715-4722.
This study aims to investigate the therapeutic effects of alkaloids in Tibetan medicine Bangna(Aconiti Penduli et Aconiti Flavi Radix) on osteoarthritis(OA) rats in vitro and in vivo and the underlying mechanisms. Chondrocytes were isolated from 2-3 week-old male SD rats and lipopolysaccharide(LPS) was used to induce OA in chondrocytes in vitro. Methyl thiazolyl tetrazolium(MTT) assay was used to investigate the toxicity of seven alkaloids(12-epi-Napelline, songorine, benzoylaconine, aconitine, 3-acetylaconitine, mesaconitine, and benzoylmesaconine) to chondrocytes. Chondrocytes were classified into the control group, model group(induced by LPS 5 mug.mL~(-1) for 12 h), and administration groups(induced by LPS 5 mug.mL~(-1) for 12 h and incubated for 24 h). The protein expression of inflammatory factors cyclooxygenase-2(COX-2), inducible nitric oxide synthetase(iNOS), tumor necrosis factor-alpha(TNF-alpha), and interleukin-1beta(IL-1beta) in each group were detected by Western blot, and the protein expression of matrix metalloprotease-13(MMP-13), aggrecan, collagen Ⅱ, fibroblast growth factor 2(FGF2) by immunofluorescence staining. For the in vivo experiment, sodium iodoacetate was used to induce OA in rats, and the expression of MMP-13, TNF-alpha, and FGF2 in cartilage tissues of rats in each group was detected by immunohistochemistry. The results showed that the viability of chondrocytes could reach more than 90% under the treatment of the seven alkaloids in a certain dose range. Aconitine, 12-epi-Napelline, songorine, 3-acetylaconitine, and mesaconitine could decrease the protein expression of inflammatory factors COX-2, iNOS, TNF-alpha and IL-1beta compared with the model group. Moreover, 12-epi-Napelline, aconitine, and mesaconitine could down-regulate the expression of MMP-13 and up-regulate the expression of aggrecan and collagen Ⅱ. In addition, compared with the model group and other Bangna alkaloids, 12-epi-Napelline significantly up-regulated the expression of FGF2. Therefore, 12-epi-Napelline was selected for the animal experiment in vivo. Immunohistochemistry results showed that 12-epi-Napelline could significantly reduce the expression of MMP-13 and TNF-alpha in cartilage tissues, and up-regulate the expression of FGF2 compared with the model group. In conclusion, among the seven Bangna alkaloids, 12-epi-Napelline can promote the repair of OA in rats by down-regulating the expression of MMP-13 and TNF-alpha and up-regulating the expression of FGF2.
Divergent Total Syntheses of Napelline-Type C20-Diterpenoid Alkaloids: (-)-Napelline, (+)-Dehydronapelline, (-)-Songorine, (-)-Songoramine, (-)-Acoapetaldine D, and (-)-Liangshanone.[Pubmed:35948501]
J Am Chem Soc. 2022 Aug 24;144(33):15355-15362.
The Napelline-type alkaloids possess an azabicyclo[3.2.1]octane moiety and an ent-kaurane-type tetracyclic skeleton (6/6/6/5) along with varied oxidation patterns embedded in the compact hexacyclic framework. Herein, we disclose a divergent entry to Napelline-type alkaloids that hinges on convergent assembly of the ent-kaurane core using a diastereoselective intermolecular Cu-mediated conjugate addition and subsequent intramolecular Michael addition reaction as well as rapid construction of the azabicyclo[3.2.1]octane motif via an intramolecular Mannich cyclization. The power of this strategy has been demonstrated through efficient asymmetric total syntheses of eight Napelline-type alkaloids, including (-)-Napelline, (-)-12-epi-Napelline, (+)-dehydroNapelline, (+)-12-epi-dehydroNapelline, (-)-songorine, (-)-songoramine, (-)-acoapetaldine D, and (-)-liangshanone.
Functional identification of the terpene synthase family involved in diterpenoid alkaloids biosynthesis in Aconitum carmichaelii.[Pubmed:34729318]
Acta Pharm Sin B. 2021 Oct;11(10):3310-3321.
Aconitum carmichaelii is a high-value medicinal herb widely used across China, Japan, and other Asian countries. Aconitine-type diterpene alkaloids (DAs) are the characteristic compounds in Aconitum. Although six transcriptomes, based on short-read next generation sequencing technology, have been reported from the Aconitum species, the terpene synthase (TPS) corresponding to DAs biosynthesis remains unidentified. We apply a combination of Pacbio isoform sequencing and RNA sequencing to provide a comprehensive view of the A. carmichaelii transcriptome. Nineteen TPSs and five alternative splicing isoforms belonging to TPS-b, TPS-c, and TPS-e/f subfamilies were identified. In vitro enzyme reaction analysis functional identified two sesqui-TPSs and twelve diTPSs. Seven of the TPS-c subfamily genes reacted with GGPP to produce the intermediate ent-copalyl diphosphate. Five AcKSLs separately reacted with ent-CPP to produce ent-kaurene, ent-atiserene, and ent-13-epi-sandaracopimaradie: a new diterpene found in Aconitum. AcTPSs gene expression in conjunction DAs content analysis in different tissues validated that ent-CPP is the sole precursor to all DAs biosynthesis, with AcKSL1, AcKSL2s and AcKSL3-1 responsible for C(20) atisine and Napelline type DAs biosynthesis, respectively. These data clarified the molecular basis for the C(20)-DAs biosynthetic pathway in A. carmichaelii and pave the way for further exploration of C(19)-DAs biosynthesis in the Aconitum species.
12-Epi-Napelline Inhibits Leukemia Cell Proliferation via the PI3K/AKT Signaling Pathway In Vitro and In Vivo.[Pubmed:34306152]
Evid Based Complement Alternat Med. 2021 Jul 1;2021:6687519.
This study aimed to investigate the inhibitory effect of 12-epi-Napelline on leukemia cells and its possible mechanisms. The inhibitory effects of 12-epi-Napelline on K-562 and HL-60 cells were evaluated using the CCK-8 assay, cell cycle arrest and apoptosis were detected by flow cytometry, and the expression of related proteins was measured by western blot. A K-562 tumor model was established to evaluate the antitumor effect of 12-epi-Napelline in vivo. A reduction in leukemia cell viability was observed after treatment with 12-epi-Napelline. It was determined that the cell cycle was arrested in the G0/G1 phase, and the cell apoptosis rate was increased. Moreover, caspase-3 and Bcl-2 were downregulated, whereas cleaved caspase-3 and caspase-9 were upregulated. Further study revealed that 12-epi-Napelline could suppress the expression of PI3K, AKT, p-AKT, and mTOR. Insulin-like growth factor 1 (IGF-1) attenuated 12-epi-Napelline-induced apoptosis and ameliorated the repression of PI3K, AKT, p-AKT, and mTOR by 12-epi-Napelline. Animal experiments clearly showed that 12-epi-Napelline inhibited tumor growth. In conclusion, 12-epi-Napelline restrained leukemia cell proliferation by suppressing the PI3K/AKT/mTOR pathway in vitro and in vivo.
Diterpenoid Alkaloids from the Aerial Parts of Aconitum flavum Hand.-Mazz.[Pubmed:33861417]
Nat Prod Bioprospect. 2021 Aug;11(4):421-429.
Sixteen diterpenoid alkaloids (DAs), including six aconitine-type alkaloids (5 and 9 - 13), seven 7,17-seco-aconitine-type alkaloids (1 - 4, 6 - 8), two Napelline-type alkaloids (14 and 15) as well as one veatchine-type alkaloid (16), were isolated from the aerial parts of Aconitum flavum Hand.-Mazz. In which, flavumolines A - D (1 - 4) were four new ones, and flavumoline E (5) was reported as natural compound for the first time. Their chemical structures were elucidated by the analysis of extensive spectroscopic data. The inhibitory activities of these isolates on Ca(v)3.1 low voltage-gated Ca(2+) channel, NO production in LPS-activated RAW264.7cells, five human tumor cell lines, as well as acetylcholinesterase (AChE) were tested.
Anti-inflammatory and anti-rheumatic activities in vitro of alkaloids separated from Aconitum soongoricum Stapf.[Pubmed:33791002]
Exp Ther Med. 2021 May;21(5):493.
The aim of the present study was to investigate the cell proliferation-inhibiting and anti-rheumatic activities of chemical components from Aconitum soongoricum Stapf. Chemical constituents of Aconitum soongoricum Stapf. were separated and purified by silica gel and Sephadex LH-20 chromatography. Structure was identified by spectroscopic technique, and physical/chemical properties were analyzed. The following four compounds were identified: i) Aconitine, ii) songorine, iii) 16, 17-dihydro-12beta, 16beta-epoxyNapelline, and iv) 12-epi-Napelline. Cell Counting kit-8 assay was performed to assess cell proliferation. ELISA was conducted to determine the cytokine contents, and reverse transcription-quantitative polymerase chain reaction and Western blot analysis were performed to detect the mRNA and protein expression levels. Compared with the lipopolysaccharide (LPS) group, the contents of IL-6, IL-1beta, TNF-alpha and PGE-2 in the culture supernatant were significantly declined in the leflunomide + LPS and intervention+LPS groups, as well as the mRNA expression levels of HIF-1alpha, VEGFA and TLR4. Treatments with songorine, benzoylaconine and aconitine (at different concentrations) significantly inhibited the proliferation of HFLS-RA cells. Compared with the LPS group, the contents of PGE-2, IL-6, IL-1beta and TNF-alpha in the culture supernatant were significantly decreased in the intervention groups, and the mRNA expression levels of TLR4, HIF-1alpha and VEGFA in the cells in the intervention groups. Songorine, benzoylaconine and aconitine from Aconitum soongoricum Stapf. have anti-rheumatic activities in vitro, which may inhibit the proliferation of HFLS-RA cells, and the underlying mechanisms may be associated with inhibiting the inflammatory cytokine production and downregulating the expression levels of HIF-1alpha, VEGF and TLR4.
Synthesis of Three-Dimensionally Fascinating Diterpenoid Alkaloids and Related Diterpenes.[Pubmed:33351595]
Acc Chem Res. 2021 Jan 5;54(1):22-34.
Three-dimensional cage-like natural products represent astounding and long-term challenges in the research endeavors of total synthesis. A central issue that synthetic chemists need to address lies in how to efficiently construct the polycyclic frameworks as well as to install the requisite substituent groups. The diterpenoid alkaloids that biogenetically originate from amination of diterpenes and diversify through late-stage skeletal reorganization belong to such a natural product category. As the characteristic components of the Aconitum and Delphinium species, these molecules display a rich array of biological activities, some of which are used as clinical drugs. More strikingly, their intricate and beautiful architectures have rendered the diterpenoid alkaloids elusive targets in the synthetic community. The successful preparation of these intriguing compounds relies on the development of innovative synthetic strategies.Our laboratory has explored the total synthesis of a variety of diterpenoid alkaloids and their biogenetically related diterpenes over the past decade. In doing so, we have accessed 6 different types of skeletons (atisine-, denudatine-, arcutane-, arcutine-, Napelline-, and hetidine-type) and achieved the total synthesis of 6 natural products (isoazitine, dihydroajaconine, gymnandine, atropurpuran, arcutinine, and liangshanone). Strategically, an oxidative dearomatization/Diels-Alder (OD/DA) cycloaddition sequence was widely employed in our synthesis to form the ubiquitous [2.2.2]-bicyclic ring unit and its related ring-distorted derivatives in these complex target molecules. This protocol, in combination with additional bond-forming key steps, allowed us to prepare the corresponding polycyclic alkaloids and a biogenetically associated diterpene. For example, bioinspired C-H activation, aza-pinacol, and aza-Prins cyclizations were used toward a unified approach to the atisine-, denudatine-, and hetidine-type alkaloids via ajaconine intermediates in our first work. To pursue the synthesis of atropurpuran and related arcutine alkaloids, we harnessed a ketyl-olefin radical cyclization to assemble the carbocycle and an aza-Wacker cyclization to construct the unusual pyrrolidine ring. Furthermore, a one-pot alkene cleavage/Mannich cyclization tactic, sequential Robinson annulation, and intramolecular aldol addition were developed, which facilitated the formation of the Napelline alkaloid scaffold and the first total synthesis of liangshanone. Finally, the utility of the Mannich cyclization and enyne cycloisomerization reactions allowed for access to the highly functionalized A/E and C/D ring fragments of aconitine (regarded as the "Holy Grail" of diterpenoid alkaloids). This Account provides insight into our synthetic designs and approaches used toward the synthesis of diterpenoid alkaloids and relevant diterpenes. These endeavors lay a foundation for uncovering the biological profiles of associated molecules and also serve as a reference for preparing other three-dimensionally fascinating natural products.
Structure, property, biogenesis, and activity of diterpenoid alkaloids containing a sulfonic acid group from Aconitum carmichaelii.[Pubmed:33163346]
Acta Pharm Sin B. 2020 Oct;10(10):1954-1965.
Three new C(20)-diterpenoid alkaloids with a sulfonic acid unit, named aconicarmisulfonines B and C (1 and 2) and chuanfusulfonine A (3), respectively, were isolated from the Aconitum carmichaelii lateral roots ("fu zi" in Chinese). Structures of 1-3 were determined by spectroscopic data analysis. Intriguing chemical properties and reactions were observed for the C(20)-diterpenoid alkaloids: (a) specific selective nucleophilic addition of the carbonyl (C-12) in 1 with CD(3)OD; (b) interconversion between 1 and 2 in D(2)O; (c) stereo- and/or regioselective deuterations of H-11alpha in 1-3 and both H-11alpha and H-11beta in aconicarmisulfonine A (4); (d) TMSP-2,2,3,3-d (4) promoted cleavage of the C-12-C-13 bond of 4 in D(2)O; (e) dehydrogenation of 4 in pyridine-d (5), and (f) Na(2)SO(3)-assisted dehydrogenation and N-deethylation of songorine (5, a putative precursor of 1-4). Biogenetically, 1 and 2 are correlated with 4, for which the same novel carbon skeleton is proposed to be derived from semipinacol rearrangements via migrations of C-13-C-16 and C-15-C-16 bonds of the Napelline-type skeleton, respectively. Meanwhile, 3 is a highly possible precursor or a concurrent product in the biosynthetic pathways of 1, 2, and 4. In the acetic acid-induced mice writhing assay, at 1.0 mg/kg (i.p.), compounds 1, 2, 5, 5a, and 5b exhibited analgesic effects against mice writhing.
Investigation of the potential anticancer effects of napelline and talatisamine dirterpenes on experimental brain tumor models.[Pubmed:32529352]
Cytotechnology. 2020 Aug;72(4):569-578.
Brain cancers are one of the most aggressive tumours in humans. Especially, gliomas are among the deadliest of human cancers and show high resistance to chemotherapeutic agents. On the other hand, discovery of biologically effective non-synthetic biomaterials in treatments of different diseases, especially cancer, has continued to be one of the most popular research topics today. Therefore, we aimed to investigate biochemical, cytological and molecular genetic effects of Napelline and talatisamine diterpenes in human U-87 MG glioma cells by using total antioxidant status and total oxidative status, 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxphenyl)-2-(4-sulfophenyl)-2H-tetrozolium, inner salt and lactate dehydrogenase release assay and RT2 Prolifer PCR Arrays. Our results revealed that Napelline and talatisamine exhibited cytotoxic effects at high doses. Napelline and talatisamine diterpenes increased apoptosis compared to control in U-87 MG cells. While Napelline induced up-regulation of 50 and down-regulation of 13 genes, talatisamine induced up-regulation of 32 and down-regulation of 18 genes in U-87 MG cells. Napelline was shown to have a higher anticancer activity than talatisamine. We think that, Napelline and talatisamine might be evaluated as potential chemotherapeutic agents for treatment of glioblastoma.
Fifteen new diterpenoid alkaloids from the roots of Aconitum kirinense Nakai.[Pubmed:31927015]
Fitoterapia. 2020 Mar;141:104477.
Extensive phytochemical investigation from the roots of Aconitum kirinense Nakai led to the identification of fifteen new compounds, including four ranaconitine type C(18)-diterpenoid alkaloids (kirisines A-D, 1-4), one lappaconitine type C(18)-diterpenoid alkaloid (kirisine E, 5), seven denudatine type C(20)-diterpenoid alkaloids (kirisines F-L, 6-12), and three Napelline type C(20)-diterpenoid alkaloids (kirisines M-O, 13-15), together with 25 known ones. Their structures were elucidated by extensive spectroscopic analyses. Among them, compounds 1 and 2 are rare diterpenoid alkaloid with 9,14-methylenedioxy group, and the latter also has a rare chloro-substituent. The diterpenoid alkaloids isolated were C(18), C(19) and C(20)-category, which might provide further clues for understanding the chemotaxonomic significance of this plant. The isolated compounds were tested for neuroprotective activity and acetylcholinesterase inhibitory activity. Compounds 7, 18, 30 and 40 which exhibited moderate activity at 80 muM against acetylcholinesterase.
Neuropharmacological effects of Aconiti Lateralis Radix Praeparata.[Pubmed:31837236]
Clin Exp Pharmacol Physiol. 2020 Apr;47(4):531-542.
Aconiti Lateralis Radix Praeparata (Fuzi in Chinese), which are the lateral roots of Aconitum Carmichaelii Debx, is widely used in China to treat many neurological diseases. Fuzi, in its various forms, has many neuropharmacological effects. It can act as an analgesic and help with depression, epilepsy, and dementia. However, the neuropharmacological effects of Aconiti Lateralis Radix Praeparata are seldom comprehensively reviewed. In this review, the neuropharmacological activities of some components contained in Aconiti Lateralis Radix Praeparata are considered. These include aconitine, mesaconitine, hypaconitine, total alkaloid, polysaccharide-1, benzoylmesaconine, fuziline, songorine, and Napelline. We also specifically discuss the antidepressant effects of total alkaloids and polysaccharide-1. This review may provide a theoretical basis for further utilization of Aconiti Lateralis Radix Praeparata for diseases that affect the central nervous system.
Diterpenoid Alkaloids and One Lignan from the Roots of Aconitum pendulum Busch.[Pubmed:31728851]
Nat Prod Bioprospect. 2019 Dec;9(6):419-423.
Diterpenoid alkaloids have neroprotective activity. Herein, three Napelline-type diterpenoid alkaloids 1-3, two aconitine-type diterpenoid alkaloids 4-5, and one isoquinline-type alkaloid 6, as well as one lignan glycoside 7, have been isolated from the roots of Aconitum pendulum Busch. Compounds 1 and 7 were new compounds, and their chemical structures were determined on the basis of nuclear magnetic resonance (NMR) spectra and mass spectrometry analysis. A ThT assay revealed that compound 2 showed significant disaggregation potency on the Abeta(1-42) aggregates.
In vivo screening of diterpene alkaloids using bdelloid rotifer assays.[Pubmed:29262708]
Acta Biol Hung. 2017 Dec;68(4):443-452.
The group of diterpene alkaloids contains numerous compounds with complex chemistry and diverse pharmacological activities. Beside toxicity, these compounds possess activity on the cardiovascular system, tumor cell lines and nervous system. The pharmacological properties have been described using in vitro and in vivo techniques; however, the bioactivities of many compounds have not thoroughly been studied. Here we report on the in vivo evaluation of ten diterpene alkaloids using bdelloid rotifer assays. Napelline exerted toxic effects on rotifers, while wide tolerance range was observed for other investigated compounds. Weak toxicity of songorine is supported by our experiment. Toxicological data for senbusine A, senbusine C, septentrioidine and hetisinone are reported for the first time.
A new denudatine type C(20)-diterpenoid alkaloid from Aconitum sinchiangense W. T. Wang.[Pubmed:29212360]
Nat Prod Res. 2018 Oct;32(19):2319-2324.
A new denudatine-type C(20)-diterpenoid alkaloid, designated as sinchianine (1), together with eight known diterpenoid alkaloids, 12-acetyl-12-epi-Napelline (2), 12-epi-Napelline (3), neoline (4), talatisamine (5), 14-O-acetylsenbusine A (6) and benzoylaconine (7), songorine (8) and aconitine (9), were isolated from the whole herb of Aconitum sinchiangense W. T. Wang. Their structures were elucidated on the basis of extensive spectroscopic analyses (NMR and HR-ESI-MS) and comparison with data reported in the literature.


