HispidolCAS# 5786-54-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 5786-54-9 | SDF | Download SDF |
| PubChem ID | 5281254.0 | Appearance | Powder |
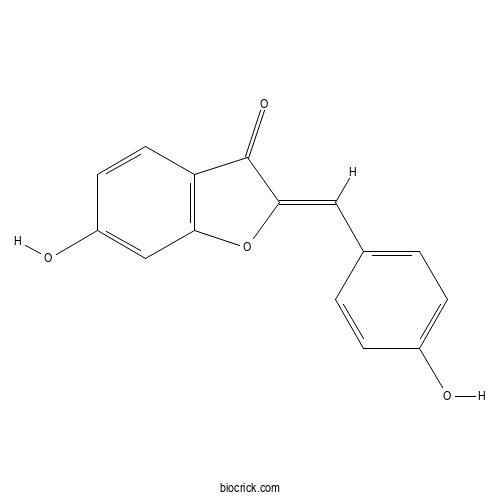
| Formula | C15H10O4 | M.Wt | 254.24 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2Z)-6-hydroxy-2-[(4-hydroxyphenyl)methylidene]-1-benzofuran-3-one | ||
| SMILES | C1=CC(=CC=C1C=C2C(=O)C3=C(O2)C=C(C=C3)O)O | ||
| Standard InChIKey | KEZLDSPIRVZOKZ-AUWJEWJLSA-N | ||
| Standard InChI | InChI=1S/C15H10O4/c16-10-3-1-9(2-4-10)7-14-15(18)12-6-5-11(17)8-13(12)19-14/h1-8,16-17H/b14-7- | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Hispidol Dilution Calculator

Hispidol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.9333 mL | 19.6665 mL | 39.3329 mL | 78.6658 mL | 98.3323 mL |
| 5 mM | 0.7867 mL | 3.9333 mL | 7.8666 mL | 15.7332 mL | 19.6665 mL |
| 10 mM | 0.3933 mL | 1.9666 mL | 3.9333 mL | 7.8666 mL | 9.8332 mL |
| 50 mM | 0.0787 mL | 0.3933 mL | 0.7867 mL | 1.5733 mL | 1.9666 mL |
| 100 mM | 0.0393 mL | 0.1967 mL | 0.3933 mL | 0.7867 mL | 0.9833 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 2',4,4',6'-Tetramethoxychalcone
Catalog No.:BCX1275
CAS No.:94103-36-3
- 2'-O-Methylphloretin
Catalog No.:BCX1274
CAS No.:111316-17-7
- Phenoxodiol
Catalog No.:BCX1273
CAS No.:81267-65-4
- 6-Hydroxyluteolin
Catalog No.:BCX1272
CAS No.:18003-33-3
- Sinomenine N-oxide
Catalog No.:BCX1271
CAS No.:1000026-77-6
- Protoanemonin
Catalog No.:BCX1270
CAS No.:108-28-1
- Demethyldaphnoretin-7-O-glucoside
Catalog No.:BCX1269
CAS No.:438578-91-7
- Scheffoleoside A
Catalog No.:BCX1268
CAS No.:160669-23-8
- 3-epi-Bufalin
Catalog No.:BCX1267
CAS No.:465-20-3
- Araloside C
Catalog No.:BCX1266
CAS No.:55446-15-6
- Dihydrolanosterol
Catalog No.:BCX1265
CAS No.:911660-54-3
- Tarasaponin IV
Catalog No.:BCX1264
CAS No.:156980-31-3
- N-Acetylcytisine
Catalog No.:BCX1277
CAS No.:6018-52-6
- Palvanil
Catalog No.:BCX1278
CAS No.:69693-13-6
- Micromarin F
Catalog No.:BCX1279
CAS No.:73292-93-0
- 3,5,7-Trimethoxyflavone
Catalog No.:BCX1280
CAS No.:26964-29-4
- Ethyl rosmarinate
Catalog No.:BCX1281
CAS No.:174591-47-0
- 1-(2,6-Dimethoxyphenyl)-3-(4-hydroxyphenyl)-2-propen-1-one
Catalog No.:BCX1282
CAS No.:85679-87-4
- 5-Hydroxy-3,7-dimethoxyflavone
Catalog No.:BCX1283
CAS No.:70786-48-0
- Morusignin L
Catalog No.:BCX1284
CAS No.:149733-95-9
- Napelline
Catalog No.:BCX1285
CAS No.:5008-52-6
- Isobellidifolin
Catalog No.:BCX1286
CAS No.:552-00-1
- Dihydrocatalpol
Catalog No.:BCX1287
CAS No.:6736-86-3
- Ajugose
Catalog No.:BCX1288
CAS No.:512-72-1
Synthesis and Biological Evaluation of O(6)-Aminoalkyl-Hispidol Analogs as Multifunctional Monoamine Oxidase-B Inhibitors towards Management of Neurodegenerative Diseases.[Pubmed:37237899]
Antioxidants (Basel). 2023 Apr 29;12(5):1033.
Oxidative catabolism of monoamine neurotransmitters by monoamine oxidases (MAOs) produces reactive oxygen species (ROS), which contributes to neuronal cells' death and also lowers monoamine neurotransmitter levels. In addition, acetylcholinesterase activity and neuroinflammation are involved in neurodegenerative diseases. Herein, we aim to achieve a multifunctional agent that inhibits the oxidative catabolism of monoamine neurotransmitters and, hence, the detrimental production of ROS while enhancing neurotransmitter levels. Such a multifunctional agent might also inhibit acetylcholinesterase and neuroinflammation. To meet this end goal, a series of aminoalkyl derivatives of analogs of the natural product Hispidol were designed, synthesized, and evaluated against both monoamine oxidase-A (MAO-A) and monoamine oxidase-B (MAO-B). Promising MAO inhibitors were further checked for the inhibition of acetylcholinesterase and neuroinflammation. Among them, compounds 3aa and 3bc were identified as potential multifunctional molecules eliciting submicromolar selective MAO-B inhibition, low-micromolar AChE inhibition, and the inhibition of microglial PGE(2) production. An evaluation of their effects on memory and cognitive impairments using a passive avoidance test confirmed the in vivo activity of compound 3bc, which showed comparable activity to donepezil. In silico molecular docking provided insights into the MAO and acetylcholinesterase inhibitory activities of compounds 3aa and 3bc. These findings suggest compound 3bc as a potential lead for the further development of agents against neurodegenerative diseases.
Positional scanning of natural product hispidol's ring-B: discovery of highly selective human monoamine oxidase-B inhibitor analogues downregulating neuroinflammation for management of neurodegenerative diseases.[Pubmed:35196956]
J Enzyme Inhib Med Chem. 2022 Dec;37(1):768-780.
Multifunctional molecules might offer better treatment of complex multifactorial neurological diseases. Monoaminergic pathways dysregulation and neuroinflammation are common convergence points in diverse neurodegenerative and neuropsychiatric disorders. Aiming to target these diseases, polypharmacological agents modulating both monoaminergic pathways and neuroinflammatory were addressed. A library of analogues of the natural product Hispidol was prepared and evaluated for inhibition of monoamine oxidases (MAOs) isoforms. Several molecules emerged as selective potential MAO B inhibitors. The most promising compounds were further evaluated in vitro for their impact on microglia viability, induced production of proinflammatory mediators and MAO-B inhibition mechanism. Amongst tested compounds, 1p was a safe potent competitive reversible MAO-B inhibitor and inhibitor of microglial production of neuroinflammatory mediators; NO and PGE(2). In-silico study provided insights into molecular basis of the observed selective MAO B inhibition. This study presents compound 1p as a promising lead compound for management of neurodegenerative disease.
Longevity effects of hispidol in Caenorhabditis elegans.[Pubmed:33179346]
Biofactors. 2020 Nov;46(6):1041-1048.
In this study, we investigated the longevity effects of Hispidol, a 6,4'-dihydroxyaurone, using the Caenorhabditis elegans model system. Our lifespan assay data revealed that Hispidol could prolong the lifespan of wild-type worms under normal culture condition. Moreover, Hispidol increased the survival rate of the worms against a heat stress condition through up-regulated expressions of HSP-16.2. Similarly, Hispidol protected worms from paraquat-induced oxidative stress. We also found that the Hispidol elevated the activities of antioxidant enzymes, thereby attenuating the generation of intracellular reactive oxygen species. These results suggest that the enhancement of lifespan and stress resistance by the Hispidol treatment might be attributed to its strong in vivo antioxidant capacity and regulation of stress proteins. Further tests on the aging-related factors revealed that Hispidol could regulate the speed of pharyngeal pumping, indicating the association of dietary restriction with the Hispidol-mediated longevity. However, there were no significant alterations in the body length of the worms between the groups. We then investigated the effects of Hispidol on body movement and lipofuscin accumulation in aged worms. Interestingly, these healthspan parameters were strongly improved by the Hispidol treatment. Our genetic studies showed no significant change in the lifespan of the daf-16 null mutants by Hispidol supplementation. In addition, enhanced nuclear translocation of DAF-16 was observed in the Hispidol-fed DAF-16::GFP fused transgenic mutants, suggesting the requirement of DAF-16/FOXO activation for the longevity effect of Hispidol.
Antidepressant-Like Activities of Hispidol and Decursin in Mice and Analysis of Neurotransmitter Monoamines.[Pubmed:32440903]
Neurochem Res. 2020 Aug;45(8):1930-1940.
The antidepressant activities of Hispidol and decursin (both potent monoamine oxidase A (MAO-A) inhibitors) were evaluated using the forced swimming test (FST) and the tail suspension test (TST) in mice, and thereafter, levels of neurotransmitter monoamines and metabolites in brain tissues were analyzed by liquid chromatography-tandem mass spectrometry (LC-MS/MS). Hispidol (15 mg/kg) caused less or comparable immobility than fluoxetine (15 mg/kg; the positive control) in immobility time, as determined by FST (9.6 vs 32.0 s) and TST (53.1 vs 48.7 s), respectively, and its effects were dose-dependent and significant. Decursin (15 mg/kg) also produced immobility comparable to that of fluoxetine as determined by FST (47.0 vs 43.4 s) and TST (55.6 vs 63.4 s), and its effects were also dose-dependent and significant. LC-MS/MS analysis after FST showed that Hispidol (15 mg/kg) greatly increased dopamine (DA) and serotonin levels dose-dependently in brain tissues as compared with the positive control. Decursin (15 mg/kg) dose-dependently increased DA level after TST. Slight changes in norepinephrine and 3,4-dihydroxyphenylacetic acid levels were observed after FST and TST in Hispidol- or decursin-treated animals. It was observed that Hispidol and decursin were effective and comparable to fluoxetine in immobility tests. These immobility and monoamine level results suggest that Hispidol and decursin are potential antidepressant agents for the treatment of depression, and that they act mainly through serotonergic and/or dopaminergic systems.
Toonamicrocarpavarin, a new tirucallane-type triterpenoid from Toona Ciliata.[Pubmed:31305146]
Nat Prod Res. 2021 Jan;35(2):266-271.
Toonamicrocarpavarin (1), a new tirucallane-type triterpenoid, along with eight known ones, piscidinol A (2), toonaciliatavarin E (3), toonayunnanin A (4), 7-acetyneotrichilenone (5), Hispidol A (6), odoratone (7), phellochin (8), toonaciliatavarin D (9), were isolated from T. ciliata. Their structures were identified on the basis of ESIMS, HREIMS and 1 D/2D NMR analysis. The cytotoxic activity of the new compound was also evaluated. All compounds were obtained from T. ciliata for the first time, which plays an important role in chemotaxonomy of the plant T. ciliata.
Effect of 25-methoxy hispidol A isolated from Poncirus trifoliate against bacteria-induced anxiety and depression by targeting neuroinflammation, oxidative stress and apoptosis in mice.[Pubmed:30583228]
Biomed Pharmacother. 2019 Mar;111:209-223.
Neuroinflammation, oxidative stress and apoptosis are implicated in the pathogenesis of neuropsychiatric diseases like anxiety and depression. 25-MethoxyHispidol A (25-MHA) is a triterpenoid isolated from the immature fruit of Poncirus trifoliate. Recently, its crude extracts have been shown to exhibit anti-bacterial and anti-inflammatory activities. The current study investigated the effect of 25-MHA (1, 5 and 10 mg/kg) against bacteria-induced anxiety and depression-like behaviors in mice. Mice were challenged intraperitoneally (i.p.) with LPS (0.83 mg/kg), S. aureus and E. coli after 30 min of 25-MHA treatment. 25-MHA (10 mg/kg) significantly mitigated the anxiety-like behavior as indicated by the results of elevated plus maze test, open field test, and light-dark box test. It also demonstrated the anti-depressant like effect by significantly reducing the immobility time in tail suspension test and forced swim test. The oxidative stress was reduced by pretreatment with 25-MHA, improving the antioxidant enzymes level such as glutathione, glutathione sulfo-transferase, and catalase. Similarly, 25-MHA attenuated the bacterial infection induced neuroinflammation by inhibiting the interleukin- 6 (IL-6), interleukin-1 beta (IL-1beta), and tumor necrosis factor-alpha (TNF-alpha) levels in prefrontal cortex and hippocampus regions. Pretreatment of 25-MHA also decreased the cortisol level and prevented changes in the thickness of the granular layer in the dentate gyrus. It also inhibited the DNA damage in hippocampus region as analyzed by comet assay. Hence, present results demonstrated that 25-MHA possesses anti-anxiety and anti-depressant activities due to the ability to reduce neuroinflammatory, oxidative stress and apoptosis induced by the administration of LPS, E. coli, and S. aureus.
Antinociceptive properties of 25-methoxy hispidol A, a triterpinoid isolated from Poncirus trifoliata (Rutaceae) through inhibition of NF-kappaB signalling in mice.[Pubmed:30456885]
Phytother Res. 2019 Feb;33(2):327-341.
The 25-methoxy Hispidol A (25-MHA) is a triterpenoid, isolated from the immature fruit of Poncirus trifoliata (Rutaceae). The pretreatment with 25-MHA markedly (p < 0.001) attenuated the formalin-induced biphasic responses as well as acetic acid-induced writhing responses. The intraperitoneal administration of 25-MHA significantly attenuated the mechanical hyperalgesia (p < 0.001) and allodynia (p < 0.05). Similarly, 25-MHA also significantly attenuated (p < 0.001) complete Freund's adjuvant (CFA)-induced paw edema in mice. The 25-MHA treatment significantly attenuated the production of nuclear kappa B (NF-kappaB) (p65 nuclear subunit). The cytokines are the important mediators of inflammation and pain; however, treatment with 25-MHA exhibited significant inhibition (p < 0.001) on the mRNA expression levels of various inflammatory mediators. The 25-MHA administration also significantly enhanced antioxidant enzymes (p < 0.001) and inhibited the oxidative stress markers. The current study indicates that 25-MHA significantly (p < 0.001) inhibited the nitric oxide (NO) in mice plasma. Similarly, the haematoxylin and eosin (H&E) staining shows that 25-MHA administration significantly inhibited the inflammatory process in the mice paw tissue compared with the CFA-treated group. The 25-MHA treatment did not exhibited any toxicity on the liver, kidney, muscles strength, and motor co-ordination in mice. The 25-MHA was coadministered with the various drugs such as tramadol, piroxicam, and gabapentin to observe the synergistic effect.
Selective inhibition of monoamine oxidase A by hispidol.[Pubmed:29395970]
Bioorg Med Chem Lett. 2018 Feb 15;28(4):584-588.
Hispidol, an aurone, isolated from Glycine max Merrill, was found to potently and selectively inhibit an isoform of recombinant human monoamine oxidase-A (MAO-A), with an IC(50) value of 0.26 microM, and to inhibit MAO-B, but with lower potency (IC(50) = 2.45 microM). Hispidol reversibly and competitively inhibited MAO-A with a K(i) value of 0.10 microM with a potency much greater than toloxatone (IC(50) = 1.10 microM), a marketed drug. It also reversibly and competitively inhibited MAO-B (K(i) = 0.51 microM). Sulfuretin, an analog of Hispidol, effectively inhibited MAO-A (IC(50) = 4.16 microM) but not MAO-B (IC(50) > 80 microM). A comparison of their chemical structures showed that the 3'-hydroxyl group of sulfuretin might reduce its inhibitory activities against MAO-A and MAO-B. Flexible docking simulation revealed that the binding affinity of Hispidol for MAO-A (-9.1 kcal/mol) was greater than its affinity for MAO-B (-8.7 kcal/mol). The docking simulation showed Hispidol binds to the major pocket of MAO-A or MAO-B. The findings suggest Hispidol is a potent, selective, reversible inhibitor of MAO-A, and that it be considered a novel lead compound for development of novel reversible inhibitors of MAO-A.
Anti-inflammatory activity of hispidol A 25-methyl ether, a triterpenoid isolated from Ponciri Immaturus Fructus.[Pubmed:19857488]
Eur J Pharmacol. 2010 Feb 10;627(1-3):318-24.
The anti-inflammatory activity of Hispidol A 25-methyl ether (Hispidol A 25-Me ether), a triterpenoid isolated from Ponciri Immaturus Fructus, was studied in lipopolysaccharide (LPS)-stimulated RAW264.7 murine macrophages. It was revealed that Hispidol A 25-Me ether dose-dependently inhibits nitric oxide (NO) production by down-regulating inducible nitric oxide synthase (iNOS). It also reduces prostaglandin E(2) (PGE(2)) production by inhibiting cyclooxygenase-2 (COX-2) expression proven on both mRNA as well as on protein levels. In addition, Hispidol A 25-Me ether inhibits mRNA expressions of major pro-inflammatory cytokines including the tumor necrosis factor-alpha (TNF-alpha), interleukin-1beta (IL-1beta) and interleukin-6 (IL-6). Interestingly, Hispidol A 25-Me ether probably exhibits a glucocorticoid-like activity, exerting functional inhibition of NF-kappaB without inhibition of DNA binging as de novo synthesis of IkappaB-alpha was induced and thereby NF-kappaB activity was reduced. Furthermore, administrations of Hispidol A 25-Me ether (1 and 10mg/kg, i.p., v/w.) were tested in two animal experiments involving acute inflammation, namely, the carrageenan-induced paw edema swelling test and the acetic acid-induced vascular permeability assay, and showed concentration-related inhibitory activities. The anti-inflammatory property of Hispidol A 25-Me ether seems to resemble the effects of the class of naturally occurring anti-inflammatory agents, glucocorticoids, which inhibit transcriptions of important inflammatory mediators.
Integrated metabolite and transcript profiling identify a biosynthetic mechanism for hispidol in Medicago truncatula cell cultures.[Pubmed:19571306]
Plant Physiol. 2009 Nov;151(3):1096-113.
Metabolic profiling of elicited barrel medic (Medicago truncatula) cell cultures using high-performance liquid chromatography coupled to photodiode and mass spectrometry detection revealed the accumulation of the aurone Hispidol (6-hydroxy-2-[(4-hydroxyphenyl)methylidene]-1-benzofuran-3-one) as a major response to yeast elicitor. Parallel, large-scale transcriptome profiling indicated that three peroxidases, MtPRX1, MtPRX2, and MtPRX3, were coordinately induced with the accumulation of Hispidol. MtPRX1 and MtPRX2 exhibited aurone synthase activity based upon in vitro substrate specificity and product profiles of recombinant proteins expressed in Escherichia coli. Hispidol possessed significant antifungal activity relative to other M. truncatula phenylpropanoids tested but has not been reported in this species before and was not found in differentiated roots in which high levels of the peroxidase transcripts accumulated. We propose that Hispidol is formed in cell cultures by metabolic spillover when the pool of its precursor, isoliquiritigenin, builds up as a result of an imbalance between the upstream and downstream segments of the phenylpropanoid pathway, reflecting the plasticity of plant secondary metabolism. The results illustrate that integration of metabolomics and transcriptomics in genetically reprogrammed plant cell cultures is a powerful approach for the discovery of novel bioactive secondary metabolites and the mechanisms underlying their generation.
Terpenoids and coumarins isolated from the fruits of Poncirus trifoliata.[Pubmed:18520091]
Chem Pharm Bull (Tokyo). 2008 Jun;56(6):839-42.
Four new triterpenes, 21alpha-methylmelianodiol (1), 21beta-methylmelianodiol (2), Hispidol A 25-methyl ether (3) and Hispidol B 25-methyl ether (4), and a new coumarin, isoschininallylol (5), were isolated from the fruits of Poncirus trifoliata RAFINESQUE, along with seventeen known compounds. The structures of the new compounds (1 - 5) were elucidated by interpretation of their spectroscopic data.
Apotirucallane and tirucallane triterpenoids from Cedrela sinensis.[Pubmed:17917286]
Chem Pharm Bull (Tokyo). 2007 Oct;55(10):1442-7.
Nine new triterpenoids, 1-9, were isolated from the cortex of Cedrela sinensis (Meliaceae), together with six known compounds, sapelin E acetate, grandifoliolenone, azadirone, bourjotinolone A, piscidinol A, and Hispidol B. The structures of 1-9 were determined by the 2D NMR experiments, chemical methods, and X-ray crystallography.
Interactions of flavones and other phytochemicals with adenosine receptors.[Pubmed:12083460]
Adv Exp Med Biol. 2002;505:163-71.
Dietary flavonoids have varied effects on animal cells, such as inhibition of platelet binding and aggregation, inhibition of inflammation, and anticancer properties, but the mechanisms of these effects remain largely unexplained. Adenosine receptors are involved in the homeostasis of the immune, cardiovascular, and central nervous systems, and adenosine agonists/antagonists exert many similar effects. The affinity of flavonoids and other phytochemicals to adenosine receptors suggests that a wide range of natural substances in the diet may potentially block the effects of endogenous adenosine. We used competitive radioligand binding assays to screen flavonoid libraries for affinity and a computational CoMFA analysis of flavonoids to compare steric and electrostatic requirements for ligand recognition at three subtypes of adenosine receptors. Flavone derivatives, such as galangin, were found to bind to three subtypes of adenosine receptors in the microM range. Pentamethylmorin (Ki 2.65 microM) was 14- to 17-fold selective for human A3 receptors than for A1 and A2A receptors. An isoflavone, genistein, was found to bind to A1 receptors. Aurones, such as Hispidol (Ki 350 nM) are selective A1 receptor antagonists, and, like genistein, are present in soy. The flavones, chemically optimized for receptor binding, have led to the antagonist, MRS 1067 (3,6-dichloro-2'-(isopropoxy)4'-methylflavone), which is 200-fold more selective for human A3 than A1 receptors. Adenosine receptor antagonism, therefore, may be important in the spectrum of biological activities reported for the flavonoids.


