ProtoanemoninCAS# 108-28-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 108-28-1 | SDF | Download SDF |
| PubChem ID | 66948.0 | Appearance | Powder |
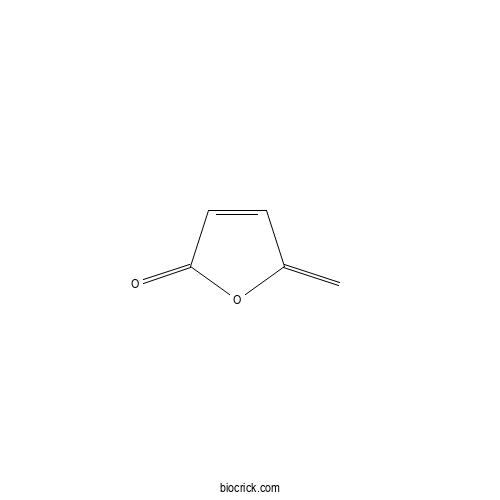
| Formula | C5H4O2 | M.Wt | 96.08 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 5-methylidenefuran-2-one | ||
| SMILES | C=C1C=CC(=O)O1 | ||
| Standard InChIKey | RNYZJZKPGHQTJR-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C5H4O2/c1-4-2-3-5(6)7-4/h2-3H,1H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Protoanemonin Dilution Calculator

Protoanemonin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 10.408 mL | 52.04 mL | 104.0799 mL | 208.1599 mL | 260.1998 mL |
| 5 mM | 2.0816 mL | 10.408 mL | 20.816 mL | 41.632 mL | 52.04 mL |
| 10 mM | 1.0408 mL | 5.204 mL | 10.408 mL | 20.816 mL | 26.02 mL |
| 50 mM | 0.2082 mL | 1.0408 mL | 2.0816 mL | 4.1632 mL | 5.204 mL |
| 100 mM | 0.1041 mL | 0.5204 mL | 1.0408 mL | 2.0816 mL | 2.602 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Demethyldaphnoretin-7-O-glucoside
Catalog No.:BCX1269
CAS No.:438578-91-7
- Scheffoleoside A
Catalog No.:BCX1268
CAS No.:160669-23-8
- 3-epi-Bufalin
Catalog No.:BCX1267
CAS No.:465-20-3
- Araloside C
Catalog No.:BCX1266
CAS No.:55446-15-6
- Dihydrolanosterol
Catalog No.:BCX1265
CAS No.:911660-54-3
- Tarasaponin IV
Catalog No.:BCX1264
CAS No.:156980-31-3
- Fallacinol
Catalog No.:BCX1263
CAS No.:569-05-1
- Isoasiaticoside
Catalog No.:BCX1262
CAS No.:948827-09-6
- Tenacissoside A
Catalog No.:BCX1261
CAS No.:107352-30-7
- cis-Pellitorine
Catalog No.:BCX1260
CAS No.:639086-18-3
- Brevicornin
Catalog No.:BCX1259
CAS No.:173792-49-9
- Stipuleanoside R2
Catalog No.:BCX1258
CAS No.:96627-72-4
- Sinomenine N-oxide
Catalog No.:BCX1271
CAS No.:1000026-77-6
- 6-Hydroxyluteolin
Catalog No.:BCX1272
CAS No.:18003-33-3
- Phenoxodiol
Catalog No.:BCX1273
CAS No.:81267-65-4
- 2'-O-Methylphloretin
Catalog No.:BCX1274
CAS No.:111316-17-7
- 2',4,4',6'-Tetramethoxychalcone
Catalog No.:BCX1275
CAS No.:94103-36-3
- Hispidol
Catalog No.:BCX1276
CAS No.:5786-54-9
- N-Acetylcytisine
Catalog No.:BCX1277
CAS No.:6018-52-6
- Palvanil
Catalog No.:BCX1278
CAS No.:69693-13-6
- Micromarin F
Catalog No.:BCX1279
CAS No.:73292-93-0
- 3,5,7-Trimethoxyflavone
Catalog No.:BCX1280
CAS No.:26964-29-4
- Ethyl rosmarinate
Catalog No.:BCX1281
CAS No.:174591-47-0
- 1-(2,6-Dimethoxyphenyl)-3-(4-hydroxyphenyl)-2-propen-1-one
Catalog No.:BCX1282
CAS No.:85679-87-4
Ranunculin, Protoanemonin, and Anemonin: Pharmacological and Chemical Perspectives.[Pubmed:38425112]
Curr Med Chem. 2024 Feb 28.
Ranunculin, a glucoside, serves as a chemotaxonomic marker in Ranunculaceae plants. When these plants are damaged, an enzyme beta-glucosidase triggers the conversion of ranunculin into Protoanemonin through hydrolysis. Subsequently, Protoanemonin undergoes cyclodimerization to form anemonin. The inherent instability of ranunculin and the rapid dimerization of Protoanemonin render them unsuitable for use in biological assays. Conversely, anemonin stands out as the optimal molecule for bioassays and demonstrates diverse biological properties, including anti-inflammatory, anti-infective, and anti-oxidant effects. Among these, anemonin exhibits the greatest promise in addressing conditions such as arthritis, cerebral ischemia, and ulcerative colitis. Its potential medical uses are enhanced by its capacity to inhibit nitric oxide synthesis and successfully counteract lipopolysaccharide-induced inflammation. This review describes the chemistry and biological properties of anemonin and its precursors, including discussions on extraction, isolation, synthesis, and investigations into bioactivity and pharmacokinetics.
Stability of protoanemonin in plant extracts from Helleborus niger L. and Pulsatilla vulgaris Mill.[Pubmed:32497949]
J Pharm Biomed Anal. 2020 Sep 5;188:113370.
The concentration levels and stability of Protoanemonin, a characteristic constituent of Ranunculaceae species with antimicrobial and fungicidal properties, were studied for the first time in plant extracts prepared from Helleborus niger L. and Pulsatilla vulgaris Mill. using fermentative production processes. Protoanemonin levels quantified by HPLC-DAD analysis were 0.0345 and 0.0662 mg/g in two freshly prepared Helleborus (whole, flowering plant) extracts and 0.3875 mg/g and 0.4193 mg/g in Pulsatilla (flowers) extracts. Protoanemonin proved to be rather instable in aqueous-fermented extracts stored at 15 degrees C in the dark, and its concentration decreased rapidly over 12 months of storage independently of the plant species. The decrease was most pronounced when initial concentrations were high (decrease by about 70%). In contrast, low Protoanemonin levels remained stable in solution for more than 12 months. Anemonin, the dimer of Protoanemonin, was detected in increasing concentrations only in Pulsatilla samples, but its concentration only accounted for less than 50% of the theoretically expected amount. With respect to fermented extracts, both physical processes such as self-polymerization and adsorption/binding to other extract constituents as well as biodegradation were concluded to be responsible for Protoanemonin decline. As opposed to plant extracts, both Protoanemonin and anemonin levels decreased in 0.22 mum-filtered samples stored in vials. This may be explained by a reduced release from plant material in combination with physicochemically induced degradation. Reduction was most pronounced upon light exposure and elevated temperatures, clearly indicating that photochemical degradation is involved. Contents of Protoanemonin in a set of extract batches were 0.0896 +/- 0.0125 mg/g and 0.0618 +/- 0.0180 mg/g in Helleborus and Pulsatilla extracts, and anemonin levels were 0.0230 +/- 0.0076 mg/g and 0.0482 +/- 0.0282 mg/g, respectively. Due to its antibiotic effects, but also its reactivity, Protoanemonin is a therapeutically and toxicologically relevant constituent, and its concentration should therefore be carefully monitored.
TianJiu therapy for alpha-naphthyl isothiocyanate-induced intrahepatic cholestasis in rats treated with fresh Ranunculus sceleratus L.[Pubmed:31629027]
J Ethnopharmacol. 2020 Feb 10;248:112310.
ETHNOPHARMACOLOGICAL RELEVANCE: TianJiu (TJ) therapy, one type of cold moxibustion, applies to specific acupuncture points with herbal patches of hot nature, providing a constant irritant to the skin until the presence of hyperemia and blistering. Traditional and clinical reports suggest that TJ is an effective therapy for the treatment of jaundice with fresh Ranunculus sceleratus L. (RS), in which Protoanemonin is one of the main irritant constituents. However, the therapeutic effect of TJ treatment with fresh RS against intrahepatic cholestasis has not been studied in animal experiments. AIM OF THE STUDY: Present study was undertaken to investigate the effect of TJ treatment with fresh RS against intrahepatic cholestasis in rats and provide an experimental basis for the underlying mechanism of TJ therapy. MATERIALS AND METHODS: Male intrahepatic cholestatic Sprague-Dawley rats induced by 2% alpha-naphthylisothiocyanate (ANIT, 80 mg/kg B.W.) were treated by TJ therapy with fresh RS. The levels of serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), direct bilirubin (DBIL), total bilirubin (TBIL), total bile acid (TBA), hepatic malondialdehyde (MDA) and nitric monoxide (NO), as well as hepatic body ratio, bile flow and hepatic histopathological assay were measured and evaluated to investigate the therapeutic effect of TJ treatment with fresh RS. Phytochemical analysis of fresh and dried RS was performed by gas chromatography-mass spectrometer (GC-MS). RESULTS: After TJ treatment with fresh RS, the abnormally elevated levels of serum AST, ALT, ALP, DBIL, TBIL and TBA, as well as hepatic MDA and NO at 108 h were reduced significantly (versus model group, P < 0.01). The hepatic body ratio, bile flow and hepatic pathological change of cholestatic rats at 108 h in TJ group were restored when compared with those of model group. Thirty-one compounds including lactones, flavonoids and phenolic acids were identified and determined by GC-MS analysis. The content of Protoanemonin in fresh RS (9.49%) was about 25-fold higher than that in dried RS (0.38%). CONCLUSIONS: TJ treatment with fresh RS exhibited good therapeutic effect on ANIT-induced intrahepatic cholestasis in rats, which may be due to the attenuated oxidative stress in the liver tissue. It is rational for the ancients to choose fresh RS as TJ herbal patches because of its abundant Protoanemonin with the character of irritant. The qualitative and quantitative results of GC-MS analysis provided the chemical basis of TJ therapy with fresh RS, which can be regarded as a simple and efficient method for the treatment of cholestasis hepatitis.
Phytocontact dermatitis due to Ranunculus arvensis: Report of three cases.[Pubmed:31180380]
North Clin Istanb. 2018 Aug 7;6(1):81-84.
Ranunculus arvensis (R. arvensis) is a member of the Ranunculaceae family and is often used by advanced age women living in rural areas for the topical treatment of arthralgia, myalgia, hemorrhoid, and warts. Protoanemonin is a substance found in the fresh leaves of the plants from the Ranunculaceae family and leads to contact dermatitis. In this report, we present three cases that developed phytocontact dermatitis caused by the topical use of R. arvensis as an alternative treatment method for arthralgia and palmoplantar psoriasis.
Preclinical evaluation of safety and potential of black hellebore extracts for cancer treatment.[Pubmed:31113420]
BMC Complement Altern Med. 2019 May 21;19(1):105.
BACKGROUND: The therapeutic use of Helleborus niger L. is manifold due to its specific phytochemical composition. Two compound groups, the ranunculin derivates including Protoanemonin and the steroidal saponins, are also associated with toxicity (genotoxicity, disintegration of membrane structures). Therefore, in vitro investigations were performed on safety aspects of a Helleborus niger aqueous fermented extract (HNE). In addition its therapeutic potential against various cancer cell lines was assessed to gain insight into the respective mechanisms of action. METHODS: To evaluate the safe use of HNE, Ames and hemolytic tests were carried out. Two angiogenesis assays in 2D and 3D design were conducted to assess the anti-angiogenetic potential, for which human umbilical vein endothelial cells (HUVEC) were chosen. A panel of tumor cell lines was used in 2D and 3D proliferation assays as well in the migration- and invasion-assay. All investigations were performed with HNE compared to reference substances. The 2D proliferation assay was additionally performed with isolated compounds of HNE (characteristic steroidal saponins). RESULTS: HNE did not exhibit any genotoxic potential. Concentrations up to 10 mul/ml were classified as non-hemolytic. HNE exerted anti-angiogenetic effects in HUVEC and anti-proliferative effects in five cancer cell lines, whereas hellebosaponin A and D as well macranthosid I did not show comparable effects neither singly nor in combination. Due to the inherent instability of Protoanemonin in isolated form, parallel investigations with Protoanemonin could not be performed. HNE (600-1000 mug/ml) inhibited the migration of certain cancer cells by > 80% such as Caki-2, DLD-1 and SK-N-SH. CONCLUSION: HNE exhibit neither genotoxic nor hemolytic potential. The present investigations verify the anti-angiogenetic effects on HUVEC, the anti-proliferative effects and migration-inhibiting properties on tumor cells. The lower effect of the relevant steroidal saponins compared to the whole extract underlines the fact that the latter is more effective than a blend of isolated pharmacologically active components.
Tunisian Clematis flammula Essential Oil Enhances Wound Healing: GC-MS Analysis, Biochemical and Histological Assessment.[Pubmed:30404969]
J Oleo Sci. 2018;67(11):1483-1499.
The aerial part of Clematis flammula (Ranunculaceae) has been traditionally used in the treatment of skin diseases including mycotic infection in the Tunisian traditional medicine. The study was undertaken to extract and determine the essential oil chemical composition of Clematis flammula aerial parts and to assess the potential of anemonin in wound healing on mechanically wounded wistar rats. The essential oil was obtained by hydrodistillation and analyzed by GC-MS. Anemonin was isolated and then incorporated as active in a cream for which the cytotoxicity was evaluated by methyl thiazolyl tetrazolium (MTT)-based colorimetric assay. Then, its potential in wound healing on mechanically wounded wistar rats was assessed. The GC-MS analysis showed that the major compound was Protoanemonin (86.74%) which spontaneously dimerised in part to form the anemonin. The wound healing activity of anemonin cream exhibited a non toxic potential of anemonin at a concentration of 25 microg/mL with a cell migration efficiency that reaches more than 80% after 48 hours of treatment. Wound healing efficiency was evaluated by monitoring morphological and skin histological analyses. Comparable wound surface reduction of the group treated by anemonin cream (p >/= 0.05) when compared to the reference treated group. The skin histological analysis showed the completely wound closure. Antioxidant activity was assessed by the malondialdehyde (MDA) rates and antioxidant enzymes (glutathione peroxidase (GPx) and catalase) determination. The results provided strong support for the effective wound healing activity of anemonin cream, making it a promising candidate as a therapeutic agent in tissue repairing processes.
The anemonin content of four different Ranunculus species.[Pubmed:30393208]
Pak J Pharm Sci. 2018 Sep;31(5(Supplementary)):2027-2032.
The Ranunculus species are poorly known as medicinal plants. They have potential toxicity given by the ranunculin and its enzymatic degradation compounds: Protoanemonin and anemonin. This paper aims to evaluate the anemonin content of four species: R. bulbosus, R. ficaria, R. sardous and R. sceleratus. The evaluation was performed by TLC and HPLC. There were evaluated two types of extracts hydroalcoholic (HA) and glycerol-ethanol (GE). The most concentrated extract in anemonin was found to be the R. sardous aerial part HA extract: 2.66 mg/ml. The lowest anemonin content is in R. sceleratus: 0.13-0.19 mg/ml. In R. bulbosus aerial part the anemonin content is less than the used HPLC method detection limits (7.68 mg/ml). In all cases the GE extracts are less concentrated in anemonin, being more safely for human administration.
Volatiles from the fungal microbiome of the marine sponge Callyspongia cf. flammea.[Pubmed:28872169]
Org Biomol Chem. 2017 Sep 13;15(35):7411-7421.
The volatiles emitted by five fungal strains previously isolated from the marine sponge Callyspongia cf. flammea were captured with a closed-loop stripping apparatus (CLSA) and analyzed by GC-MS. Besides several widespread compounds, a series of metabolites with interesting bioactivities were found, including the quorum sensing inhibitor Protoanemonin, the fungal phytotoxin 3,4-dimethylpentan-4-olide, and the insect attractant 1,2,4-trimethoxybenzene. In addition, the aromatic polyketides isotorquatone and chartabomone that are both known from Eucalyptus and a new O-desmethyl derivative were identified. The biosynthesis of isotorquatone was studied by feeding experiments with isotopically labeled precursors and its absolute configuration was determined by enantioselective synthesis of a reference compound. Bioactivity testings showed algicidal activity for some of the identified compounds, suggesting a potential ecological function in sponge defence.
A rare chemical burn due to Ranunculus arvensis: three case reports.[Pubmed:26922695]
Ann Saudi Med. 2016 Jan-Feb;36(1):89-91.
Ranunculus arvensis, a plant that is a member of Ranunculaceae family, generally used for local treatment of joint pain, muscle pain, burns, lacerations, edema, abscess drainage, hemorrhoids, and warts among the population. In this case report, we presented three patients who developed chemical skin burns after using R. arvensis plant locally for knee pain. The destructive effect of the plant has been reported previously to be more in fresh plants and less in dried plants. Although Protoanemonin, which is considered as the main toxic substance, was reported to be absent in dried or boiled plants, the plant was boiled, cooled, and wrapped over the region with pain in our cases. Therefore, we thought that Protoanemonin may be considered to be heat resistant. Also, the burn management proceeded up to surgery by using the flap technique in one of our patients in contrast to the cases found in published reports who were treated by antibiotics and dressings.
Differential cytotoxic properties of Helleborus niger L. on tumour and immunocompetent cells.[Pubmed:25446603]
J Ethnopharmacol. 2015 Jan 15;159:129-36.
ETHNOPHARMACOLOGICAL RELEVANCE: In Romanian folk medicine, Helleborus niger L. is used for the treatment of rheumatoid arthritis or viral infections and in complementary therapy, especially in anthroposophic medicine (AM), where the plant is administered as an adjuvant to treat malignant diseases. In the present study, we investigated the differential cytotoxic effects of H. niger on human tumour and healthy cells of the human immune system in vitro. MATERIAL AND METHODS: Protoanemonin and saponins, as significant constituents of H. niger extracts, were quantified in five individual batches using validated HPLC methods. Further, the impact of H. niger on proliferation capacity (MTT assay) as well as on apoptosis and necrosis induction in a panel of tumour cell lines and human lymphocytes (combined annexin V and propidium iodide staining) was monitored. In addition, NK cell function (degranulation-CD107a assay and IFN-gamma secretion) was also investigated since these immunocompetent cells are important for the control of malignancies within the human body. RESULTS: Extracts of H. niger induced proliferation inhibition not only of lymphoblastic leukaemia cells (MOLT4; IC50: 171 microg/mL) but also of myosarcoma (SK-UT-1b; IC50: 304 microg/mL) and melanoma cells (HT-144; IC50: 569 microg/mL) due to the induction of apoptosis. Purified T cells or NK cells were significantly affected through the presence of high H. niger concentrations while bulk lymphocytes were not affected. NK cells' anti-tumour functions expressed by degranulation capacity as well as IFN-y production were unaffected by the presence of the H. niger extract. Since Protoanemonin and saponins have been reported in the literature to exert cytotoxic effects, their content was also determined. CONCLUSIONS: H. niger extracts exhibit differential cytotoxicity towards tumour cell lines and healthy human T- and NK-cells.
Comprehensive study of the phenolics and saponins from Helleborus niger L. Leaves and stems by liquid chromatography/tandem mass spectrometry.[Pubmed:24591317]
Chem Biodivers. 2014 Feb;11(2):276-98.
The aerial parts of the medicinal plant Helleborus niger L. comprise a substantial number of constituents with only few of them identified so far. To expand the knowledge of its secondary metabolite profile, extracts from H. niger leaves and stems were investigated by liquid chromatography/tandem mass spectrometry (LC/MS(n) ). Specific identification strategies using LC/MS are established and discussed in detail. The leaves turned out to contain acylated and non-acylated quercetin and kaempferol oligoglycosides, Protoanemonin and its precursor ranunculin, beta-ecdysone, and a variety of steroidal saponins, mainly in the furostanol form. The sapogenins were elucidated as of sarsasapogenyl, diosgenyl, and macranthogenyl structures, and confirmed by comparison with the respective reference compounds. The secondary metabolite profiles were almost identical in both plant parts except that the stems lacked kaempferol derivatives and some saponins. The ranunculin derivatives and beta-ecdysone were found in both plant parts. Correlations between the location of the compound groups and the plant's defense strategies are proposed. Additionally, the role of the detected secondary metabolites as protective substances against exogenic stress and as a defense against herbivores is discussed.
New branches in the degradation pathway of monochlorocatechols by Aspergillus nidulans: a metabolomics analysis.[Pubmed:24509097]
J Hazard Mater. 2014 Mar 15;268:264-72.
A collective view of the degradation of monochlorocatechols in fungi is yet to be attained, though these compounds are recognised as key degradation intermediates of numerous chlorinated aromatic hydrocarbons, including monochlorophenols. In the present contribution we have analysed the degradation pathways of monochlorophenols in Aspergillus nidulans using essentially metabolomics. Degradation intermediates herein identified included those commonly reported (e.g. 3-chloro-cis,cis-muconate) but also compounds never reported before in fungi revealing for 4-chlorocatechol and for 3-chlorocatechol unknown degradation paths yielding 3-chlorodienelactone and catechol, respectively. A different 3-chlorocatechol degradation path led to accumulation of 2-chloromuconates (a potential dead-end), notwithstanding preliminary evidence of chloromuconolactones and Protoanemonin simultaneous formation. In addition, some transformation intermediates, of which sulfate conjugates of mono-chlorophenols/chlorocatechols were the most common, were also identified. This study provides critical information for understanding the role of fungi in the degradation of chlorinated aromatic hydrocarbons; furthering their utility in the development of innovative bioremediation strategies.


