PhenoxodiolCAS# 81267-65-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 81267-65-4 | SDF | Download SDF |
| PubChem ID | 219100.0 | Appearance | Powder |
| Formula | C15H12O3 | M.Wt | 240.26 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
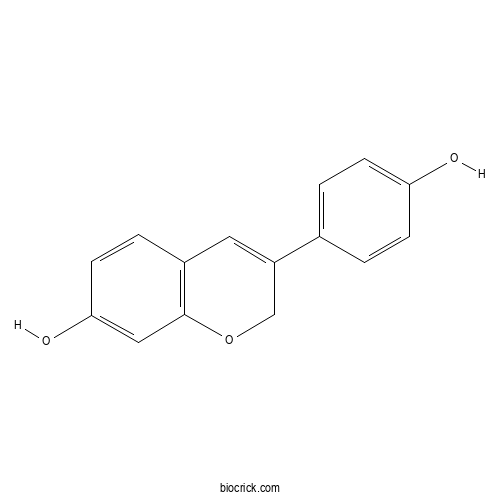
| Chemical Name | 3-(4-hydroxyphenyl)-2H-chromen-7-ol | ||
| SMILES | C1C(=CC2=C(O1)C=C(C=C2)O)C3=CC=C(C=C3)O | ||
| Standard InChIKey | ZZUBHVMHNVYXRR-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C15H12O3/c16-13-4-1-10(2-5-13)12-7-11-3-6-14(17)8-15(11)18-9-12/h1-8,16-17H,9H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Phenoxodiol Dilution Calculator

Phenoxodiol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.1622 mL | 20.8108 mL | 41.6216 mL | 83.2432 mL | 104.0539 mL |
| 5 mM | 0.8324 mL | 4.1622 mL | 8.3243 mL | 16.6486 mL | 20.8108 mL |
| 10 mM | 0.4162 mL | 2.0811 mL | 4.1622 mL | 8.3243 mL | 10.4054 mL |
| 50 mM | 0.0832 mL | 0.4162 mL | 0.8324 mL | 1.6649 mL | 2.0811 mL |
| 100 mM | 0.0416 mL | 0.2081 mL | 0.4162 mL | 0.8324 mL | 1.0405 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 6-Hydroxyluteolin
Catalog No.:BCX1272
CAS No.:18003-33-3
- Sinomenine N-oxide
Catalog No.:BCX1271
CAS No.:1000026-77-6
- Protoanemonin
Catalog No.:BCX1270
CAS No.:108-28-1
- Demethyldaphnoretin-7-O-glucoside
Catalog No.:BCX1269
CAS No.:438578-91-7
- Scheffoleoside A
Catalog No.:BCX1268
CAS No.:160669-23-8
- 3-epi-Bufalin
Catalog No.:BCX1267
CAS No.:465-20-3
- Araloside C
Catalog No.:BCX1266
CAS No.:55446-15-6
- Dihydrolanosterol
Catalog No.:BCX1265
CAS No.:911660-54-3
- Tarasaponin IV
Catalog No.:BCX1264
CAS No.:156980-31-3
- Fallacinol
Catalog No.:BCX1263
CAS No.:569-05-1
- Isoasiaticoside
Catalog No.:BCX1262
CAS No.:948827-09-6
- Tenacissoside A
Catalog No.:BCX1261
CAS No.:107352-30-7
- 2'-O-Methylphloretin
Catalog No.:BCX1274
CAS No.:111316-17-7
- 2',4,4',6'-Tetramethoxychalcone
Catalog No.:BCX1275
CAS No.:94103-36-3
- Hispidol
Catalog No.:BCX1276
CAS No.:5786-54-9
- N-Acetylcytisine
Catalog No.:BCX1277
CAS No.:6018-52-6
- Palvanil
Catalog No.:BCX1278
CAS No.:69693-13-6
- Micromarin F
Catalog No.:BCX1279
CAS No.:73292-93-0
- 3,5,7-Trimethoxyflavone
Catalog No.:BCX1280
CAS No.:26964-29-4
- Ethyl rosmarinate
Catalog No.:BCX1281
CAS No.:174591-47-0
- 1-(2,6-Dimethoxyphenyl)-3-(4-hydroxyphenyl)-2-propen-1-one
Catalog No.:BCX1282
CAS No.:85679-87-4
- 5-Hydroxy-3,7-dimethoxyflavone
Catalog No.:BCX1283
CAS No.:70786-48-0
- Morusignin L
Catalog No.:BCX1284
CAS No.:149733-95-9
- Napelline
Catalog No.:BCX1285
CAS No.:5008-52-6
The role of isoflavones in augmenting the effects of radiotherapy.[Pubmed:36936272]
Front Oncol. 2023 Mar 1;12:800562.
Cancer is one of the major health problems and the second cause of death worldwide behind heart disease. The traditional soy diet containing isoflavones, consumed by the Asian population in China and Japan has been identified as a protective factor from hormone-related cancers. Over the years the research focus has shifted from emphasizing the preventive effect of isoflavones from cancer initiation and promotion to their efficacy against established tumors along with chemo- and radiopotentiating effects. Studies performed in mouse models and results of clinical trials emphasize that genistein or a mixture of isoflavones, containing in traditional soy diet, could be utilized to both potentiate the response of cancer cells to radiotherapy and reduce radiation-induced toxicity in normal tissues. Currently ongoing clinical research explores a potential of another significant isoflavone, idronoxil, also known as Phenoxodiol, as radiation enhancing agent. In the light of the recent clinical findings, this article reviews the accumulated evidence which support the clinically desirable interactions of soy isoflavones with radiation therapy resulting in improved tumor treatment. This review discusses important aspects of the development of isoflavones as anticancer agents, and mechanisms potentially relevant to their activity in combination with radiation therapy of cancer. It gives a critical overview of studies characterizing isoflavone targets such as topoisomerases, ENOX2/PMET, tyrosine kinases and ER receptor signaling, and cellular effects on the cell cycle, DNA damage, cell death, and immune responses.
Enzymatic synthesis of novel unnatural phenoxodiol glycosides with a glycosyl donor flexible glycosyltransferase MeUGT1.[Pubmed:35998478]
Enzyme Microb Technol. 2022 Nov;161:110113.
Isoflavonoids are of great interest due to their human health-promoting properties, which have resulted in studies on exploiting these phytochemicals as hotspots in diverse bio -industries. Biocatalytic glycosylation of isoflavonoid aglycones to glycosides has attracted marked interests because it enable the biosynthesis of isoflavonoid glycosides with high selectivity under mild conditions, and also provide an environmentally friendly option for the chemical synthesis. Thus, these inspired us to exploit new flexible and effective glycosyltransferases from microbes for making glycosides attractive compounds that are in high demand in several industries. Most recently, we have reported the functional characterization of a bacterial-origin recombinant glycosyltransferase (MeUGT1). Herein, more detailed kinetic characteristics of this biocatalyst, using a number of glycosyl donor substrates, were examined for further investigation of its biocatalytic applicability, enabling it feasible to biosynthesize new glycosides; Phenoxodiol-4'-O-alpha-glucuronide, Phenoxodiol-4'-O-alpha-(2''-N-acetyl)glucosaminide, Phenoxodiol-4'-O-alpha-galactoside, Phenoxodiol-4'-O-alpha-(2''-N-acetyl)galactosaminide and Phenoxodiol-4'-O-alpha-(2''-deoxy)glucoside. The thorough kinetic analyses revealed that while the recombinant enzyme can utilize, albeit with different substrate preference and catalytic efficiency, a total five different nucleotide sugars as glycosyl donors, exhibiting its promiscuity towards glycosyl donors. This is the first report that a recombinant glycosyltransferase MeUGT1 that can regio-specifically glycosylate C4'-hydroxyl function of semi-synthetic Phenoxodiol isoflavene to biosynthesize a series of unnatural Phenoxodiol-4'-O-alpha-glycosides.
Glycosylation of Semi-Synthetic Isoflavene Phenoxodiol with a Recombinant Glycosyltransferase from Micromonospora echinospora ATCC 27932.[Pubmed:35131959]
J Microbiol Biotechnol. 2022 May 28;32(5):657-662.
Glycosyltransferase (GT)-specific degenerate PCR screening followed by in silico sequence analyses of the target clone was used to isolate a member of family1 GT-encoding genes from the established fosmid libraries of soil actinomycetes Micromonospora echinospora ATCC 27932. A recombinant MeUGT1 was heterologously expressed as a His-tagged protein in E. coli, and its enzymatic reaction with semi-synthetic Phenoxodiol isoflavene (as a glycosyl acceptor) and uridine diphosphate-glucose (as a glycosyl donor) created two different glycol-attached products, thus revealing that MeUGT1 functions as an isoflavonoid glycosyltransferase with regional flexibility. Chromatographic separation of product glycosides followed by the instrumental analyses, clearly confirmed these previously unprecedented glycosides as Phenoxodiol-4'-alpha-O-glucoside and Phenoxodiol-7-alpha-O-glucoside, respectively. The antioxidant activities of the above glycosides are almost the same as that of parental Phenoxodiol, whereas their anti-proliferative activities are all superior to that of cisplatin (the most common platinum chemotherapy drug) against two human carcinoma cells, ovarian SKOV-3 and prostate DU-145. In addition, they are more water-soluble than their parental aglycone, as well as remaining intractable to the simulated in vitro digestion test, hence demonstrating the pharmacological potential for the enhanced bio-accessibility of Phenoxodiol glycosides. This is the first report on the microbial enzymatic biosynthesis of Phenoxodiol glucosides.
The potential anti-amyloidogenic candidate, SPA1413, for Alzheimer's disease.[Pubmed:34610141]
Br J Pharmacol. 2022 Mar;179(5):1033-1048.
BACKGROUND AND PURPOSE: Recently, isoflavone derivatives have been shown to have neuroprotective effects against neurological disorders. For instance, genistein attenuated the neuroinflammation and amyloid-beta accumulation in Alzheimer's disease animal models, suggesting the potential for use to prevent and treat Alzheimer's disease. EXPERIMENTAL APPROACH: Here, 50 compounds, including isoflavone derivatives, were constructed and screened for the inhibitory effects on amyloid-beta(42) fibrilization and oligomerization using the high-throughput screening formats of thioflavin T assay and multimer detection system, respectively. The potential neuroprotective effect of t3-(4-hydroxyphenyl)-2H-chromen-7-ol (SPA1413), also known as dehydroequol, idronoxil or Phenoxodiol, was evaluated in cells and in 5xFAD (B6SJL) transgenic mouse, a model of Alzheimer's disease. KEY RESULTS: SPA1413 had a potent inhibitory action on both amyloid-beta fibrilization and oligomerization. In the cellular assay, SPA1413 prevented amyloid-beta-induced cytotoxicity and reduced neuroinflammation. Remarkably, the oral administration of SPA1413 ameliorated cognitive impairment, decreased amyloid-beta plaques and activated microglia in the brain of 5xFAD (B6SJL) transgenic mouse. CONCLUSION AND IMPLICATIONS: Our results strongly support the repurposing of SPA1413, which has already received fast-track status from the US Food and Drug Administration (FDA) for cancer treatment, for the treatment of Alzheimer's disease due to its potent anti-amyloidogenic and anti-neuroinflammatory actions.
Flavonoids and Related Members of the Aromatic Polyketide Group in Human Health and Disease: Do They Really Work?[Pubmed:32847100]
Molecules. 2020 Aug 24;25(17):3846.
Some aromatic polyketides such as dietary flavonoids have gained reputation as miraculous molecules with preeminent beneficial effects on human health, for example, as antioxidants. However, there is little conclusive evidence that dietary flavonoids provide significant leads for developing more effective drugs, as the majority appears to be of negligible medicinal importance. Some aromatic polyketides of limited distribution have shown more interesting medicinal properties and additional research should be focused on them. Combretastatins, analogues of Phenoxodiol, hepatoactive kavalactones, and silymarin are showing a considerable promise in the advanced phases of clinical trials for the treatment of various pathologies. If their limitations such as adverse side effects, poor water solubility, and oral inactivity are successfully eliminated, they might be prime candidates for the development of more effective and in some case safer drugs. This review highlights some of the newer compounds, where they are in the new drug pipeline and how researchers are searching for additional likely candidates.
Phenoxodiol sensitizes metastatic colorectal cancer cells to 5-fluorouracil- and oxaliplatin-induced apoptosis through intrinsic pathway.[Pubmed:32665777]
EXCLI J. 2020 Jun 30;19:936-949.
Colorectal cancer (CRC) is one of the most common types of cancer seen in the world. 5-Fluorouracil (5-Fu) plus Oxaliplatin (1-OHP) remains the backbone of CRC chemotherapeutics, but with limited success. Phenoxodiol (Pxd) is an isoflavone analog with antitumor activity against various types of cancers, and sensitizes chemoresistant cancer cells to chemotherapeutics including platinum and taxanes. This study was, therefore, undertaken to examine whether Pxd pre-treatment with conventional chemotherapeutic agent(s) 5-Fu and 1-OHP co-administration be a therapeutic strategy for CRC. Cell viability and cytotoxicity were evaluated using dimethyl-thiazolyl diphenyl tetrazolium bromide (MTT) and lactate dehydrogenase assays. The percentage of apoptotic and necrotic cells were determined by fluorescence microscopy analysis. Besides, active Caspase-3 levels by ELISA and relative mRNA levels of Caspase 3 (CASP3), CASP8 and CASP9 genes were determined by quantitative real-time PCR (qPCR) analysis. The pre-treatment of Pxd followed by 5-Fu and 1-OHP co-administration was more effective at inhibiting cell viability than either chemotherapeutic agents treatment alone. When compared to 5-Fu with 1-OHP alone treatment, Pxd pre-treatment overwhelmingly increased apoptotic Caspase-3 activity levels in CRC cells. Moreover, qPCR analyses showed that CASP3 and CASP9 mRNA levels significantly increased after pre-treatment with Pxd followed by 5-Fu and 1-OHP treatments, compared to 5-Fu with 1-OHP alone. Our results suggested that Pxd enhanced the in vitro antitumor activity of 5-Fu and 1-OHP. Our study also suggested that Pxd may be a potential candidate agent in advanced CRC and inclusion of Pxd to the conventional chemotherapeutic agent(s) could be an effective therapeutic strategy for CRC.
African mustard (Brassica tournefortii) as source of nutrients and nutraceuticals properties.[Pubmed:32476145]
J Food Sci. 2020 Jun;85(6):1856-1871.
Brassica tournefortii is an annual herbaceous plant, native to the North Africa and Middle East. It is considered as an excellent medicinal plant due to its richness by antioxidant like isothiocyanates and polyphenols. The present study is the first phytochemical investigation on Brassica tournefortii organs (leaves, stems, and roots) in terms of nutraceutical, chemical composition, and bioactivity. Brassica tournefortii leaves exhibited the highest values of nutraceutical contents. Interestingly, gas chromatograph-y-mass spectrometry (GC-MS) analysis enabled to identify three new isothiocyanates: iberverin nitrile and iberin detected only in roots, and iberin nitrile detected in all organs. HPLC chromatograms displayed different profiles depending on organic solvent and extracted organ. Icariin and 5,7-dihydroxy 4-propylcoumarin showed the highest concentrations with 2.3 and 1.3 mg/g of dr among other molecules identified by high performance liquid chromatography (HPLC). Some phenolic compounds were identified in more than one organ extracts such as Phenoxodiol and 4-hydroxy-3-propylbenzoic acid methyl ester. Brassica tournefortii extracts showed a moderate total phenolic contents and anti-15-LOX activity, while they exhibited a good anti-alpha-glucosidase activity ranging from 40% to 60%. Furthermore, leaves-MeOH and root-dichloromethane (DCM) extracts induced the highest cytotoxicity against MCF-7 cell lines, while roots-cyclohexane (CYHA) extract highlighted the highest inhibition activity against, both, HCT-116 and OVCAR cell lines.
Synthesis of Dextran-Phenoxodiol and Evaluation of Its Physical Stability and Biological Activity.[Pubmed:31440502]
Front Bioeng Biotechnol. 2019 Aug 8;7:183.
Phenoxodiol, an isoflavene anti-tumor agent, was conjugated on the polysaccharide dextran using immobilized laccase as biocatalyst. The success of the enzymatic conjugation was determined by UV-vis spectrophotometry and its functionalization degree was assessed by (1)H NMR and was found to be 3.25 mg Phenoxodiol/g of conjugate. An accelerated stability test showed that the resultant conjugate was nine times more stable than the free Phenoxodiol when tested for its residual anti-oxidant activity with the Folin-Ciocalteu assay. The in vitro anti-proliferative activity of the conjugate was evaluated against neuroblastoma SKN-BE(2)C, triple-negative breast cancer MDA-MB-231, and glioblastoma U87 cancer cells. The conjugate was shown to be generally more potent than Phenoxodiol against all three cell types tested. Additionally, the cytotoxicity and anti-angiogenic activity of the conjugate were also evaluated against non-malignant human lung fibroblast MRC-5 and human microvascular endothelial cells HMEC-1, respectively. The conjugate was found to be 1.5 times less toxic than Phenoxodiol while mostly retaining 62% of its anti-angiogenic activity in the conjugate form. This study provides further evidence that the conjugation of natural product-derived drugs onto polysaccharide molecules such as dextran can lead to better stability and enhanced biological activity of the conjugate compared to the free drug alone.
Correction to: Triggering of eryptosis, the suicidal erythrocyte death, by phenoxodiol.[Pubmed:31332477]
Naunyn Schmiedebergs Arch Pharmacol. 2019 Oct;392(10):1319.
The original version of this article contains several mistakes due to the missed corrections.
Triggering of eryptosis, the suicidal erythrocyte death, by phenoxodiol.[Pubmed:31280326]
Naunyn Schmiedebergs Arch Pharmacol. 2019 Oct;392(10):1311-1318.
Phenoxodiol is used for the treatment of malignancy. The substance is effective by triggering suicidal tumor cell death or apoptosis. At least in theory, Phenoxodiol could similarly stimulate suicidal erythrocyte death or eryptosis. Eryptosis is characterized by cell shrinkage and breakdown of cell membrane asymmetry with phosphatidylserine translocation to the erythrocyte surface. Signaling of eryptosis includes increase of cytosolic Ca(2+) activity ([Ca(2+)](i)), formation of reactive oxygen species (ROS), and increase of ceramide abundance at the cell surface. The present study explored whether Phenoxodiol induces eryptosis and whether it modifies Ca(2+) entry, ROS, and ceramide. Using flow cytometry, phosphatidylserine exposure at the cell surface was quantified from annexin V binding, cell volume from forward scatter, [Ca(2+)](i) from Fluo3 fluorescence, ROS from DCFDA-dependent fluorescence, and ceramide abundance utilizing specific antibodies. A 48-h exposure of human erythrocytes to Phenoxodiol (100 mug/ml [416 muM]) significantly increased the percentage of annexin V binding cells, significantly decreased average forward scatter and Fluo3 fluorescence and significantly increased ceramide abundance, but did not significantly modify DCFDA fluorescence. The effect of Phenoxodiol on annexin V binding tended to decrease following removal of extracellular Ca(2+), an effect, however, not reaching statistical significance. In conclusion, Phenoxodiol triggers eryptosis, an effect paralleled by increase of ceramide abundance.
Pharmacology of ME-344, a novel cytotoxic isoflavone.[Pubmed:30885362]
Adv Cancer Res. 2019;142:187-207.
Isoflavones isolated from members of the Fabaceae (primarily Leguminosae) family have been characterized for their phytoestrogenic properties, but certain derivatives have also shown potential as possible cancer therapeutic agents. ME-344, related to Phenoxodiol (Fig. 1), is a second generation isoflavone with a recent history of both preclinical and early clinical testing. The drug has unusual cytotoxicity profiles, where cancer cell lines can be categorized as either intrinsically sensitive or resistant to the drug. Evolving studies show that the cytotoxic properties of the drug are enacted through targeting mitochondrial bioenergetics. While the drug has undergone early Phase I/II trials in solid tumors with confined dose limiting effects and some evidence of disease response, there is a continuing need to define specific cellular targets that determine sensitivity, with the long-term goal of applying such information to individualized therapy. This review article details some of the existing and ongoing studies that are assisting in the continued drug development processes that may lead to new drug application (NDA) status.
Evaluation of Therapeutic Potential of Phenoxodiol, a Novel Isoflavone Analog, in Renal Cancer Cells.[Pubmed:30275191]
Anticancer Res. 2018 Oct;38(10):5709-5716.
BACKGROUND/AIM: In the present study, the antineoplastic activity and mechanism of action of Phenoxodiol, a novel isoflavone analog, was investigated in renal cancer cells. MATERIALS AND METHODS: A panel of renal cancer cells (769-P, 786-O, Caki-2) was treated with Phenoxodiol in vitro, and the efficacy of treatment was evaluated. RESULTS: MTS assay results showed that Phenoxodiol decreased renal cancer viability in a dose-dependent manner. In addition, it inhibited colony formation significantly and perturbed the cell cycle. Treatment with Phenoxodiol increased the number of annexin-V-positive cells as well as the expression of cleaved poly ADP ribose polymerase, demonstrating that Phenoxodiol induced apoptosis in renal cancer cells. Phenoxodiol also inhibited Akt pathway via dephosphorylation of Akt. CONCLUSION: Phenoxodiol inhibited Akt pathway and induced apoptosis of renal cancer cells. The present study provides a theoretical basis for future development of a novel therapy effective against renal cancer.
Phenoxodiol Increases Cisplatin Sensitivity in Ovarian Clear Cancer Cells Through XIAP Down-regulation and Autophagy Inhibition.[Pubmed:29277787]
Anticancer Res. 2018 Jan;38(1):301-306.
BACKGROUND/AIM: To investigate whether XIAP down-regulation and autophagy inhibition sensitize ovarian clear cell cancer cells to cisplatin. MATERIALS AND METHODS: The ovarian clear cancer cell line KK was used for in vitro analysis. Hydroxychloroquine (HCQ) and Phenoxodiol (PXD) or embelin were used as autophagy and XIAP inhibitors, respectively. Non-specific and XIAP-specific siRNAs were transfected using Lipofectamine. Cytotoxicity was assessed by MTT assays. Protein expression was confirmed by western blotting. RESULTS: In KK, down-regulation of XIAP using specific siRNAs together with HCQ treatment enhanced the anti-tumor effect of cisplatin. Although embelin sensitized KK to cisplatin through XIAP down-regulation, it induced autophagy. However, PXD increased cisplatin sensitivity through XIAP down-regulation and autophagy inhibition. Expression of Atg7, Atg12, and Beclin 1 was decreased after PXD treatment. CONCLUSION: PXD increased cisplatin sensitivity through XIAP down-regulation and autophagy inhibition and could be a new candidate for ovarian clear cell carcinoma treatment.
Synthesis of isoflavene-thiosemicarbazone hybrids and evaluation of their anti-tumor activity.[Pubmed:28408225]
Bioorg Med Chem Lett. 2017 Jun 1;27(11):2454-2458.
Phenoxodiol is an isoflavene with potent anti-tumor activity. In this study, a series of novel mono- and di-substituted Phenoxodiol-thiosemicarbazone hybrids were synthesized via the condensation reaction between Phenoxodiol with thiosemicarbazides. The in vitro anti-proliferative activities of the hybrids were evaluated against the neuroblastoma SKN-BE(2)C, the triple negative breast cancer MDA-MB-231, and the glioblastoma U87 cancer cell lines. The mono-substituted hybrids exhibited potent anti-proliferative activity against all three cancer cell lines, while the di-substituted hybrids were less active. Selected mono-substituted hybrids were further investigated for their cytotoxicity against normal MRC-5 human lung fibroblast cells, which identified two hybrids with superior selectivity for cancer cells over normal cells as compared to Phenoxodiol. This suggests that mono-substituted Phenoxodiol-thiosemicarbazone hybrids have promising potential for further development as anti-cancer agents.


