Theasaponin E1CAS# 220114-28-3 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 220114-28-3 | SDF | Download SDF |
| PubChem ID | 9920037.0 | Appearance | Powder |
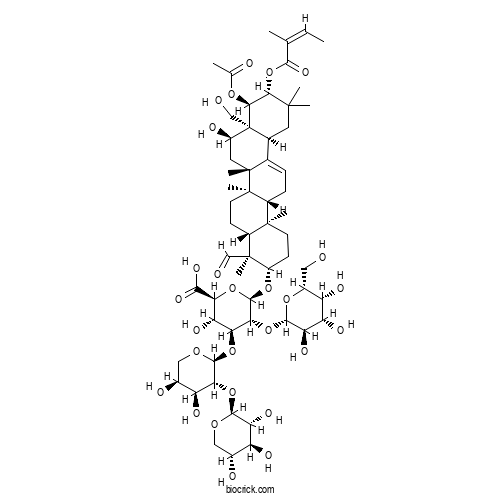
| Formula | C59H90O27 | M.Wt | 1231.34 |
| Type of Compound | Triterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2S,3S,4S,5R,6R)-6-[[(3S,4S,4aR,6aR,6bS,8R,8aR,9R,10R,12aS,14aR,14bR)-9-acetyloxy-4-formyl-8-hydroxy-8a-(hydroxymethyl)-4,6a,6b,11,11,14b-hexamethyl-10-[(Z)-2-methylbut-2-enoyl]oxy-1,2,3,4a,5,6,7,8,9,10,12,12a,14,14a-tetradecahydropicen-3-yl]oxy]-4-[(2S,3R,4S,5S)-4,5-dihydroxy-3-[(2S,3R,4S,5R)-3,4,5-trihydroxyoxan-2-yl]oxyoxan-2-yl]oxy-3-hydroxy-5-[(2S,3R,4S,5R,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyoxane-2-carboxylic acid | ||
| SMILES | CC=C(C)C(=O)OC1C(C2(C(CC1(C)C)C3=CCC4C5(CCC(C(C5CCC4(C3(CC2O)C)C)(C)C=O)OC6C(C(C(C(O6)C(=O)O)O)OC7C(C(C(CO7)O)O)OC8C(C(C(CO8)O)O)O)OC9C(C(C(C(O9)CO)O)O)O)C)CO)OC(=O)C | ||
| Standard InChIKey | WWVKOCDDDWJQLC-MWQJAWBESA-N | ||
| Standard InChI | InChI=1S/C59H90O27/c1-10-24(2)49(76)86-46-47(79-25(3)63)59(23-62)27(17-54(46,4)5)26-11-12-32-55(6)15-14-34(56(7,22-61)31(55)13-16-57(32,8)58(26,9)18-33(59)66)81-53-45(85-51-40(72)38(70)37(69)30(19-60)80-51)42(41(73)43(83-53)48(74)75)82-52-44(36(68)29(65)21-78-52)84-50-39(71)35(67)28(64)20-77-50/h10-11,22,27-47,50-53,60,62,64-73H,12-21,23H2,1-9H3,(H,74,75)/b24-10-/t27-,28+,29-,30+,31+,32+,33+,34-,35-,36-,37-,38-,39+,40+,41-,42-,43-,44+,45+,46-,47-,50-,51-,52-,53+,55-,56-,57+,58+,59-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Theasaponin E1 Dilution Calculator

Theasaponin E1 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 0.8121 mL | 4.0606 mL | 8.1212 mL | 16.2425 mL | 20.3031 mL |
| 5 mM | 0.1624 mL | 0.8121 mL | 1.6242 mL | 3.2485 mL | 4.0606 mL |
| 10 mM | 0.0812 mL | 0.4061 mL | 0.8121 mL | 1.6242 mL | 2.0303 mL |
| 50 mM | 0.0162 mL | 0.0812 mL | 0.1624 mL | 0.3248 mL | 0.4061 mL |
| 100 mM | 0.0081 mL | 0.0406 mL | 0.0812 mL | 0.1624 mL | 0.203 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Kakkanin
Catalog No.:BCX1301
CAS No.:63770-91-2
- 16α-hydroxy-3-oxo-lanosta-7,9(11),24-trien-21-oic acid
Catalog No.:BCX1300
CAS No.:862109-64-6
- Xylopine
Catalog No.:BCX1299
CAS No.:517-71-5
- Lyciumin A
Catalog No.:BCX1298
CAS No.:125708-06-7
- Kanshone C
Catalog No.:BCX1297
CAS No.:117634-64-7
- Hypoglaunine D
Catalog No.:BCX1296
CAS No.:220751-00-8
- Amaroswerin
Catalog No.:BCX1295
CAS No.:21233-18-1
- t-OMe-Byakangelicin
Catalog No.:BCX1294
CAS No.:79638-04-3
- Ajugasterone C 2-acetate
Catalog No.:BCX1293
CAS No.:154510-93-7
- Chaparrinone
Catalog No.:BCX1292
CAS No.:22611-34-3
- Physcion-8-O-β-gentiobioside
Catalog No.:BCX1291
CAS No.:84268-38-2
- Wistin
Catalog No.:BCX1290
CAS No.:19046-26-5
- Lyciumin B
Catalog No.:BCX1303
CAS No.:125756-66-3
- Poststerone
Catalog No.:BCX1304
CAS No.:10162-99-9
- Corymbiferin
Catalog No.:BCX1305
CAS No.:5042-09-1
- Shinjulactone M
Catalog No.:BCX1306
CAS No.:103630-27-9
- 13α,21-Dihydroeurycomanone
Catalog No.:BCX1307
CAS No.:129587-06-0
- Entadamide A
Catalog No.:BCX1308
CAS No.:100477-88-1
- 3'-O-Angeloylhamaudol
Catalog No.:BCX1309
CAS No.:84272-84-4
- Theasaponin E2
Catalog No.:BCX1310
CAS No.:220114-30-7
- Oxypeucedanin hydrate-3”-ethyl ether
Catalog No.:BCX1311
CAS No.:55481-87-3
- Isooxypeucedanin
Catalog No.:BCX1312
CAS No.:5058-15-1
- Poricoic acid BM
Catalog No.:BCX1313
CAS No.:1815623-74-5
- Neoartanin
Catalog No.:BCX1314
CAS No.:104196-69-2
Anti-Biofilm Activity of Assamsaponin A, Theasaponin E1, and Theasaponin E2 against Candida albicans.[Pubmed:38612411]
Int J Mol Sci. 2024 Mar 22;25(7):3599.
Biofilm formation plays a crucial role in the pathogenesis of Candida albicans and is significantly associated with resistance to antifungal agents. Tea seed saponins, a class of non-ionic triterpenes, have been proven to have fungicidal effects on planktonic C. albicans. However, their anti-biofilm activity and mechanism of action against C. albicans remain unclear. In this study, the effects of three Camellia sinensis seed saponin monomers, namely, Theasaponin E1 (TE1), theasaponin E2 (TE2), and assamsaponin A (ASA), on the metabolism, biofilm development, and expression of the virulence genes of C. albicans were evaluated. The results of the XTT reduction assay and crystal violet (CV) staining assay demonstrated that tea seed saponin monomers concentration-dependently suppressed the adhesion and biofilm formation of C. albicans and were able to eradicate mature biofilms. The compounds were in the following order in terms of their inhibitory effects: ASA > TE1 > TE2. The mechanisms were associated with reductions in multiple crucial virulence factors, including cell surface hydrophobicity (CSH), adhesion ability, hyphal morphology conversion, and phospholipase activity. It was further demonstrated through qRT-PCR analysis that the anti-biofilm activity of ASA and TE1 against C. albicans was attributed to the inhibition of RAS1 activation, which consequently suppressed the cAMP-PKA and MAPK signaling pathways. Conversely, TE2 appeared to regulate the morphological turnover and hyphal growth of C. albicans via a pathway that was independent of RAS1. These findings suggest that tea seed saponin monomers are promising innovative agents against C. albicans.
Anti-Candida albicans Effects and Mechanisms of Theasaponin E1 and Assamsaponin A.[Pubmed:37298302]
Int J Mol Sci. 2023 May 27;24(11):9350.
Candida albicans is an opportunistic human fungal pathogen, and its drug resistance is becoming a serious problem. Camellia sinensis seed saponins showed inhibitory effects on resistant Candida albicans strains, but the active components and mechanisms are unclear. In this study, the effects and mechanisms of two Camellia sinensis seed saponin monomers, Theasaponin E1 (TE1) and assamsaponin A (ASA), on a resistant Candida albicans strain (ATCC 10231) were explored. The minimum inhibitory concentration and minimum fungicidal concentration of TE1 and ASA were equivalent. The time-kill curves showed that the fungicidal efficiency of ASA was higher than that of TE1. TE1 and ASA significantly increased the cell membrane permeability and disrupted the cell membrane integrity of C. albicans cells, probably by interacting with membrane-bound sterols. Moreover, TE1 and ASA induced the accumulation of intracellular ROS and decreased the mitochondrial membrane potential. Transcriptome and qRT-PCR analyses revealed that the differentially expressed genes were concentrated in the cell wall, plasma membrane, glycolysis, and ergosterol synthesis pathways. In conclusion, the antifungal mechanisms of TE1 and ASA included the interference with the biosynthesis of ergosterol in fungal cell membranes, damage to the mitochondria, and the regulation of energy metabolism and lipid metabolism. Tea seed saponins have the potential to be novel anti-Candida albicans agents.
Neuroprotective Effects of Green Tea Seed Isolated Saponin Due to the Amelioration of Tauopathy and Alleviation of Neuroinflammation: A Therapeutic Approach to Alzheimer's Disease.[Pubmed:35408478]
Molecules. 2022 Mar 24;27(7):2079.
Tauopathy is one of the major causes of neurodegenerative disorders and diseases such as Alzheimer's disease (AD). Hyperphosphorylation of tau proteins by various kinases leads to the formation of PHF and NFT and eventually results in tauopathy and AD; similarly, neuroinflammation also exaggerates and accelerates neuropathy and neurodegeneration. Natural products with anti-tauopathy and anti-neuroinflammatory effects are highly recommended as safe and feasible ways of preventing and /or treating neurodegenerative diseases, including AD. In the present study, we isolated Theasaponin E1 from ethanol extract of green tea seed and evaluated its therapeutic inhibitory effects on tau hyper-phosphorylation and neuroinflammation in neuroblastoma (SHY-5Y) and glioblastoma (HTB2) cells, respectively, to elucidate the mechanism of the inhibitory effects. The expression of tau-generating and phosphorylation-promoting genes under the effects of Theasaponin E1 were determined and assessed by RT- PCR, ELISA, and western blotting. It was found that Theasaponin E1 reduced hyperphosphorylation of tau and Abeta concentrations significantly, and dose-dependently, by suppressing the expression of GSK3 beta, CDK5, CAMII, MAPK, EPOE4(E4), and PICALM, and enhanced the expression of PP1, PP2A, and TREM2. According to the ELISA and western blotting results, the levels of APP, Abeta, and p-tau were reduced by treatment with Theasaponin E1. Moreover, Theasaponin E1 reduced inflammation by suppressing the Nf-kB pathway and dose-dependently reducing the levels of inflammatory cytokines such as IL-1beta, IL-6, and TNF-alpha etc.
Pharmacological Approaches to Attenuate Inflammation and Obesity with Natural Products Formulations by Regulating the Associated Promoting Molecular Signaling Pathways.[Pubmed:34812408]
Biomed Res Int. 2021 Nov 12;2021:2521273.
Obesity is a public health problem characterized by increased body weight due to abnormal adipose tissue expansion. Bioactive compound consumption from the diet or intake of dietary supplements is one of the possible ways to control obesity. Natural products with adipogenesis-regulating potential act as obesity treatments. We evaluated the synergistic antiangiogenesis, antiadipogenic and antilipogenic efficacy of standardized rebaudioside A, sativoside, and Theasaponin E1 formulations (RASE1) in vitro in human umbilical vein endothelial cells (HUVECs), 3T3-L1 preadipocytes respectively, and in vivo using a high-fat and carbohydrate diet-induced obesity mouse model. Orlistat was used as a positive control, while untreated cells and animals were normal controls (NCs). Adipose tissue, liver, and blood were analyzed after dissection. Extracted stevia compounds and green tea seed saponin E1 exhibited pronounced antiobesity effects when combined. RASE1 inhibited HUVEC proliferation and tube formation by suppressing VEGFR2, NF-kappaB, PIK3, and-catenin beta-1 expression levels. RASE1 inhibited 3T3-L1 adipocyte differentiation and lipid accumulation by downregulating adipogenesis- and lipogenesis-promoting genes. RASE1 oral administration reduced mouse body and body fat pad weight and blood cholesterol, TG, ALT, AST, glucose, insulin, and adipokine levels. RASE1 suppressed adipogenic and lipid metabolism gene expression in mouse adipose and liver tissues and enhanced AMP-activated protein kinase levels in liver and adipose tissues and in serum adiponectin. RASE1 suppressed the NF-kappaB pathway and proinflammatory cytokines IL-10, IL-6, and TNF-alpha levels in mice which involve inflammation and progression of obesity. The overall results indicate RASE1 is a potential therapeutic formulation and functional food for treating or preventing obesity and inflammation.
Green Tea Seed Isolated Theasaponin E1 Ameliorates AD Promoting Neurotoxic Pathogenesis by Attenuating Abeta Peptide Levels in SweAPP N2a Cells.[Pubmed:32429462]
Molecules. 2020 May 16;25(10):2334.
Alzheimer's disease (AD) is the most frequent type of dementia affecting memory, thinking and behaviour. The major hallmark of the disease is pathological neurodegeneration due to abnormal aggregation of Amyloid beta (Abeta) peptides generated by beta- and gamma-secretases via amyloidogenic pathway. Purpose of the current study was to evaluate the effects of Theasaponin E1 on the inhibition of Abeta producing beta-, gamma-secretases (BACE1, PS1 and NCT) and acetylcholinesterase and activation of the non-amyloidogenic APP processing alpha-secretase (ADAM10). Additionally, Theasaponin E1 effects on Abeta degrading and clearing proteins neprilysin and insulin degrading enzyme (IDE). The effect of Theasaponin E1 on these crucial enzymes was investigated by RT-PCR, ELISA, western blotting and fluorometric assays using mouse neuroblastoma cells (SweAPP N2a). Theasaponin E1 was extracted and purified from green tea seed extract via HPLC, and N2a cells were treated with different concentrations for 24 h. Gene and protein expression in the cells were measured to determine the effects of activation and/or inhibition of Theasaponin E1 on beta- and gamma-secretases, neprilysin and IDE. Results demonstrated that Theasaponin E1 significantly reduced Abeta concentration by activation of the alpha-secretase and neprilysin. The activities of beta- and gamma-secretase were reduced in a dose-dependent manner due to downregulation of BACE1, presenilin, and nicastrin. Similarly, Theasaponin E1 significantly reduced the activity of acetylcholinesterase. Overall, from the results it is concluded that green tea seed extracted saponin E1 possess therapeutic significance as a neuroprotective natural product recommended for the treatment of Alzheimer's disease.
Preservative effect of Camellia sinensis (L.) Kuntze seed extract in soy sauce and its mutagenicity.[Pubmed:29433297]
Food Res Int. 2018 Mar;105:982-988.
The purpose of this study was to investigate the applicability of green tea seed (GTS) extract as a natural preservative in food. Food preservative ability and mutagenicity studies of GTS extract and identification of antimicrobial compounds from GTS extract were carried out. The GTS extract showed only anti-yeast activity against Candida albicans with MIC value of 938mug/mL and Zygosaccharomyces rouxii with a MIC of 469mug/mL. The active compounds were identified as Theasaponin E1 (1), assamsaponin A (2), and assamsaponin B (3). And GTS extracts didn't show mutagenicity because there were no dose-dependent changes in colonies of Salmonella typhimurium TA98, TA100, TA1535, TA1537, and Escherichia coli WP(2)uvrA regardless of the metabolic activation system. And GTS extract also showed a potent food preservation affect which eliminated all yeast below the MIC value in application test at soy sauce. Overall, these results indicate that GTS extract could be a safe and effective food preservative with anti-yeast activity.
Phytochemical analysis of the triterpenoids with cytotoxicity and QR inducing properties from the total tea seed saponin of Camellia sinensis.[Pubmed:23266730]
Fitoterapia. 2013 Jan;84:321-5.
The tea seed triterpene saponin (TS) from Camellia sinensis was found to exhibit better antitumor activity in vivo in S180 implanted ICR mice and QR inducing activity for hepa lclc7 cells respectively compared with the total tea seed saponin (TTS), hydrolysate of the TTS and tea seed flavonoid glycosides (TF). By bioassay-guided isolation, the TS fraction was separated and seven major components were purified and identified as Theasaponin E1 (1), theasaponin E2 (2), theasaponin C1 (3), assamsaponin C (4), theasaponin H1 (5), theasaponin A9 (6), and theasaponin A8 (7), among which compounds 4 and 5 were isolated from this genus for the first time. The antitumor bioassay of the isolated compounds showed that compounds 1, 2 and 3 exhibited potential activities against the human tumor cell lines K562 and HL60. Furthermore, compound 1 (the major constituent with a mass content of over 1%) showed significant QR inducing activity with an IR value of 4.2 at 4mug/ml. So it can be concluded that tea seed especially the compound 1 (Theasaponin E1) could be used as an antitumor agent and a chemoprevention agent of cancer. The preliminary structure-activity relationship in the anti-tumor activity and QR inducing activity of tea saponins was discussed briefly.
Theasaponin E1 destroys the salt tolerance of yeasts.[Pubmed:16232924]
J Biosci Bioeng. 2000;90(6):637-42.
Cells of Zygosaccharomyces rouxii in a medium containing a high concentration of NaCl were killed during incubation for 2-4 h with a low concentration of a mixture of saponins from tea seeds (TSS). The higher the concentration of NaCl in the medium, the higher the inhibitory effect of TSS on the growth of the yeast. The above inhibitory effect of TSS on the growth of the yeast was not observed when cells were incubated in hypertonic media composed of nonionic substances such as sugars. The ATPase activity of plasma membrane preparations from the yeast cells was slightly affected by the addition of TSS. It is shown that TSS facilitates leakage of glycerol from the yeast cells under NaCl-hypertonic conditions. The major inhibitor in the mixture of saponins was isolated and identified as Theasaponin E1. Its isomer, theasaponin E2, did not have any effect on the salt tolerance of Z. rouxii or Saccharomyces cerevisiae.
Bioactive saponins and glycosides. XVII. Inhibitory effect on gastric emptying and accelerating effect on gastrointestinal transit of tea saponins: structures of assamsaponins F, G, H, I, and J from the seeds and leaves of the tea plant.[Pubmed:11086901]
Chem Pharm Bull (Tokyo). 2000 Nov;48(11):1720-5.
Following the investigation of assamsaponins A, B, C, D, and E, four new saponins termed assamsaponins F, G, H, and I were isolated from the seeds of the tea plant (Camellia sinensis L. var. assamica PIERRE), while assamsaponin J was isolated from its leaves. The structures of assamsaponins F-J were elucidated on the basis of chemical and physicochemical evidence and found to be 16,22-O-diacetyl-21-O-angeloyltheasapogenol E 3-O-[beta-D-galactopyranosyl (1-->2)][beta-D-glucopyranosyl(1 -->2)- alpha-L-arabinopyranosyl(1-->3)]-beta-D-glucopyranosiduronic acid, 21-O-angeloyl-22-O-acetyltheasapogenol E 3-O-[beta-D-galactopyranosyl(1--> 2)][beta-D-glucopyranosyl(1-->2)-alpha-L-arabinopyranosyl(1-->3)]- beta-D-glucopyranosiduronic acid, 21-O-angeloyl-28-O-acetyltheasapogenol E 3-O-[beta-D-galactopyranosyl(1-->2)][beta-D-glucopyranosyl(1--> 2)-alpha-L-arabinopyranosyl(1-->3)]-beta-D-glucopyranosiduronic acid, 21-O-tigloyl-28-O-acetyltheasapogenol E 3-O-[beta-D-galactopyranosyl(1--> 2)][beta-D-glucopyranosyl(1--> 2)-alpha-L-arabinopyranosyl(1-->3)]-beta-D-glucopyranosiduronic acid, and 16,21-O-diacetyl-22-O-cinnamoyltheasapogenol B 3-O-[beta-D-galactopyranosyl(l-->2)][beta-D-rhamnopyranosy(1-->2)- alpha-L-arabinopyranosyl(1-->3)]-beta-D-glucopyranosiduronic acid, respectively. The saponin mixture from the seeds of the tea plant was found to exhibit an inhibitory effect on gastric emptying and an accelerating effect on gastrointestinal transit in mice. Theasaponin E1 the principle saponin of the tea plant, showed potent activity, while theasaponin E2 showed none, so that the position of the acyl groups in the sapogenin moiety is important from a pharmacological point of view.


