Quercetin-3'-glucosideCAS# 19254-30-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 19254-30-9 | SDF | Download SDF |
| PubChem ID | 5748594 | Appearance | Powder |
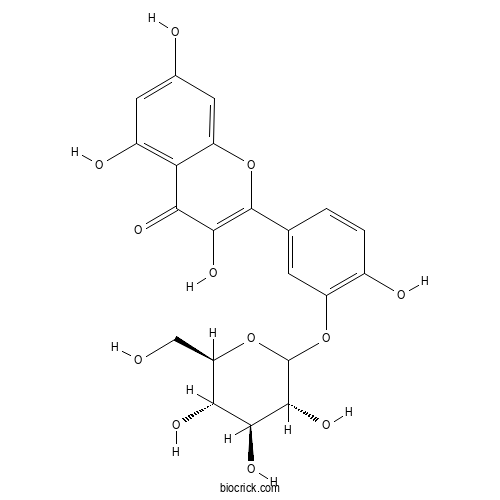
| Formula | C21H20O12 | M.Wt | 464.4 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 3,5,7-trihydroxy-2-[4-hydroxy-3-[(3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyphenyl]chromen-4-one | ||
| SMILES | C1=CC(=C(C=C1C2=C(C(=O)C3=C(C=C(C=C3O2)O)O)O)OC4C(C(C(C(O4)CO)O)O)O)O | ||
| Standard InChIKey | YLWQTYZKYGNKPI-CXWQUDHASA-N | ||
| Standard InChI | InChI=1S/C21H20O12/c22-6-13-15(26)17(28)19(30)21(33-13)32-11-3-7(1-2-9(11)24)20-18(29)16(27)14-10(25)4-8(23)5-12(14)31-20/h1-5,13,15,17,19,21-26,28-30H,6H2/t13-,15-,17+,19-,21?/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Quercetin-3'-glucoside Dilution Calculator

Quercetin-3'-glucoside Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1533 mL | 10.7666 mL | 21.5332 mL | 43.0663 mL | 53.8329 mL |
| 5 mM | 0.4307 mL | 2.1533 mL | 4.3066 mL | 8.6133 mL | 10.7666 mL |
| 10 mM | 0.2153 mL | 1.0767 mL | 2.1533 mL | 4.3066 mL | 5.3833 mL |
| 50 mM | 0.0431 mL | 0.2153 mL | 0.4307 mL | 0.8613 mL | 1.0767 mL |
| 100 mM | 0.0215 mL | 0.1077 mL | 0.2153 mL | 0.4307 mL | 0.5383 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 1,3-Diphenyl-2-propen-1-one
Catalog No.:BCN9986
CAS No.:94-41-7
- 4'-Methoxyflavanone
Catalog No.:BCN9985
CAS No.:97005-76-0
- Farnesol
Catalog No.:BCN9984
CAS No.:4602-84-0
- (R,S)-Equol
Catalog No.:BCN9983
CAS No.:66036-38-2
- 4',6,7-Trimethoxyisoflavone
Catalog No.:BCN9982
CAS No.:798-61-8
- trans-Beta-Apo-8'-carotenal
Catalog No.:BCN9981
CAS No.:1107-26-2
- (-)-Verbenone
Catalog No.:BCN9980
CAS No.:1196-01-6
- Harmol
Catalog No.:BCN9979
CAS No.:487-03-6
- Henricine
Catalog No.:BCN9978
CAS No.:107783-46-0
- omega-Benzoyl oxyphloracetophenone
Catalog No.:BCN9977
CAS No.:65982-77-6
- AT101
Catalog No.:BCN9976
CAS No.:866541-93-7
- 9-Methyl-9-azabicyclo[3.3.1]nonan-3-one
Catalog No.:BCN9975
CAS No.:552-70-5
- trans-Aconitic acid
Catalog No.:BCN9988
CAS No.:4023-65-8
- 3-Octyl alcohol
Catalog No.:BCN9989
CAS No.:589-98-0
- Phloroglucinol aldehyde triethylether
Catalog No.:BCN9990
CAS No.:59652-88-9
- Daclatasvir
Catalog No.:BCN9991
CAS No.:1009119-64-5
- Geranylacetate
Catalog No.:BCN9992
CAS No.:105-87-3
- Cytochalasin C
Catalog No.:BCN9993
CAS No.:22144-76-9
- 4-Hydroxyquinoline
Catalog No.:BCN9994
CAS No.:611-36-9
- 2',3,5,7-Tetrahydroxyflavone
Catalog No.:BCN9995
CAS No.:480-15-9
- 3,4-Dimethoxychalcone
Catalog No.:BCN9996
CAS No.:5416-71-7
- Oxyacanthine hydrochloride
Catalog No.:BCN9997
CAS No.:15352-74-6
- 7-Methoxyflavone
Catalog No.:BCN9998
CAS No.:22395-22-8
- trans-5-Hydroxyferulic acid
Catalog No.:BCN9999
CAS No.:110642-42-7
[Antidepressant activity of flavonoid ethanol extract of Abelmoschus manihot corolla with BDNF up-regulation in the hippocampus].[Pubmed:29979503]
Yao Xue Xue Bao. 2017 Feb;52(2):222-8.
Abelmoschus manihot (L.) Medic., a folk herbal medicine in China, is a flowering plant belonging to Abelmoschus L. genus and Malvaceae family, which has been reported with an antidepressant activity. The study was designed to isolate flavonoids from Abelmoschus manihot corolla and explore the action mechanism of antidepressant activities. The flavonoids were isolated and purified by D101 macroporous resin column, polyamide column and Sephadex LH-20 sequentially and identified as myricetin-3-O-beta-D-glucoside (1), gossypetin-8-O-beta-D-glucuronide (2, G-8-G), gossypetin-3'-O-beta-D-glucoside (3), quercetin-3'-glucoside (4, Q-3-G), isoquercitrin (5, IQT), hyperoside (6, HY), myricetin (7), quercetin (8, QT). Compounds 2, 4, 5, 6 and 8 (15, 30 and 60 mg.kg-1) were orally administered to mice and the reaction was observed in tail suspension test (TST) and forced swimming test (FST). Western blot analysis was used in determination of the protein expressions of brain-derived neurotrophic factor (BDNF), tyrosine receptor kinase B (TrkB) and phosphorylation eukaryotic elongation factor 2 (p-eEF2). The results revealed that only Q-3-G and G-8-G (15, 30, 60 mg .kg-1) significantly reduced the immobility time in FST and TST. Furthermore, Q-3-G and G-8-G remarkably increased the expression of BDNF and TrkB, and decreased the expression of p-eEF2. These results suggest that Q-3-G and G-8-G had an obvious antidepressant activity via up-regulation of BDNF expression. The new observation will provide a new direction in the development of antidepressant in the treatment of major depressive disorder (MDD).
A new lyoniresinol derivative from Smilax microphylla.[Pubmed:23472472]
Nat Prod Commun. 2013 Jan;8(1):113-4.
A new lignan, lyoniresinol-9-O-8"-syringylglycerol ether (1), together with five known compounds, piceatannol (2), resveratrol (3), oxyresveratrol (4), quercetin-3'-glucoside (5) and diosgenin (6) were isolated from the rhizomes of Smilax microphylla. The structure of the new compound was determined by means of chemical evidence and 1D-and 2D-NMR (1H, 13C, HSQC, HMBC, 1H-1H COSY and NOESY) spectroscopic analysis and HR-ESI-MS.
Flavonoids possess neuroprotective effects on cultured pheochromocytoma PC12 cells: a comparison of different flavonoids in activating estrogenic effect and in preventing beta-amyloid-induced cell death.[Pubmed:17323972]
J Agric Food Chem. 2007 Mar 21;55(6):2438-45.
Despite the classical hormonal effect, estrogen possesses a neuroprotective effect in the brain, which has led many to search for novel treatments for neurodegenerative diseases. Flavonoids, a group of compounds mainly derived from vegetables, share a resemblance, chemically, to estrogen, and indeed, some have been used as estrogen substitutes. To search for potential therapeutic agents against neurodegenerative diseases, different subclasses of flavonoids were analyzed and compared with estrogen. First, the estrogenic activities of these flavonoids were determined by activating the estrogen-responsive elements in cultured MCF-7 breast cancer cells. Second, the neuroprotective effects of flavonoids were revealed by measuring its inhibition effects on the formation of reactive oxygen species, the aggregation of beta-amyloid, and the induction of cell death by beta-amyloid in cultured neuronal PC12 cells. Among these flavonoids, baicalein, scutellarin, hibifolin, and quercetin-3'-glucoside possessed the strongest effect in neuroprotection; however, the neuroprotective activity did not directly correlate with the estrogenic activity of the flavonoids. Identification of these flavonoids could be very useful in finding potential drugs, or food supplements, for treating Alzheimer's disease.


