RO5126766(CH5126766)Raf/MEK dual inhibitor CAS# 946128-88-7 |
- Trametinib (GSK1120212)
Catalog No.:BCC1282
CAS No.:871700-17-3
- Vemurafenib (PLX4032, RG7204)
Catalog No.:BCC1269
CAS No.:918504-65-1
- PLX-4720
Catalog No.:BCC1280
CAS No.:918505-84-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 946128-88-7 | SDF | Download SDF |
| PubChem ID | 16719221 | Appearance | Powder |
| Formula | C21H18FN5O5S | M.Wt | 471.46 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | CH5126766 | ||
| Solubility | DMSO : 100 mg/mL (212.11 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
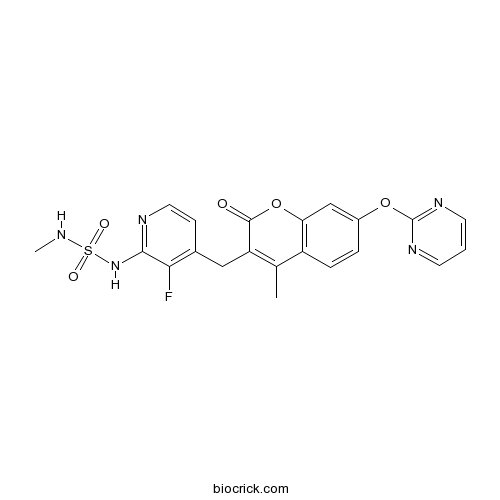
| Chemical Name | 3-[[3-fluoro-2-(methylsulfamoylamino)pyridin-4-yl]methyl]-4-methyl-7-pyrimidin-2-yloxychromen-2-one | ||
| SMILES | CC1=C(C(=O)OC2=C1C=CC(=C2)OC3=NC=CC=N3)CC4=C(C(=NC=C4)NS(=O)(=O)NC)F | ||
| Standard InChIKey | LMMJFBMMJUMSJS-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C21H18FN5O5S/c1-12-15-5-4-14(31-21-25-7-3-8-26-21)11-17(15)32-20(28)16(12)10-13-6-9-24-19(18(13)22)27-33(29,30)23-2/h3-9,11,23H,10H2,1-2H3,(H,24,27) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Ro 5126766 is a first-in-class dual MEK/RAF inhibitor that allosterically inhibits BRAFV600E, CRAF, MEK, and BRAF (IC50: 8.2, 56, 160 nM, and 190 nM, respectively).In Vitro:Ro 5126766 (RO5126766) is an allosteric inhibitor that binds directly to MEK and prevents its phosphorylation by RAF through the formation of a stable RAF-MEK complex. Ro 5126766 inhibits both the phosphorylation of MEK by RAF and the activation of ERK by MEK. In cell-free MEK and RAF kinase assays, Ro 5126766 effectively inhibits activation of ERK2 by MEK1 with an IC50 of 160 nM (SD=±0.043) and inhibits the phosphorylation of MEK1 protein by BRAF (IC50=190 nM, SD=±0.003), BRAFV600E (IC50=8.2 nM, SD=±0.0015), and CRAF (IC50=56 nM, SD=±0.016). Ro 5126766 effectively inhibits both MEK and ERK phosphorylation in a panel of human tumor cell lines including KRAS/HRAS and BRAF mutant cell lines and KRAS/HRAS and BRAF wild-type cells[1]. In order to investigate whether the mevalonate pathway affects the sensitivity to MEK inhibitors, human breast cancer MDA-MB-231 cells harboring KRAS and BRAF mutations are treated Ro 5126766 (CH5126766), with or without statins, which inhibits HMG-CoA reductase, the rate-limiting enzyme in the mevalonate pathway. The combined treatment of Ro 5126766 with Fluvastatin demonstrates more significant reduction in cell growth in a dose-dependent manner than the single treatment of Ro 5126766. The marked combined effects of Ro 5126766 at 40 nM and Fluvastatin at 0.3 μM is also confirmed on the suppression of the colony formation of the cells[2].In Vivo:In KRAS-mutant xenograft models, Ro 5126766 (RO5126766) inhibits growth and causes tumor regressions more effectively than another allosteric MEK inhibitor, PD0325901. Preclinical data from a series of human tumor mouse xenograft models indicates an ED50 for Ro 5126766 of 0.03 to 0.23 mg/kg and an ED90 of 0.15 to 1.56 mg/kg. These effective doses are associated with target trough concentrations of 17 to 133 ng/L and 87 to 901 ng/mL, respectively. [1]. In this experiment, Ro 5126766 (CH5126766) or PD0325901 is administrated at their maximum tolerated dose (MTD) in the HCT116 model (1.5 and 25 mg/kg, respectively). These doses inhibit pERK and ERK signaling output at similar degrees in the tumors from the drug-treated mice at 4 hours from the first drug administration. Moreover, in HCT116 models, the ED50 for Ro 5126766 and PD0325901 are 0.056 and 0.80 mg/kg, respectively. Therefore, the doses used for this experiment are 26.8- and 31.3-fold higher doses than the 50% effective doses, respectively. Daily oral administration of either drug causes significant tumor regression of each these tumors. However, whereas inhibition of tumor growth is maintained for the entire 28-day treatment period in Ro 5126766-treated mice, tumor models receiving PD0325901 become refractory after 10 days of treatment[3]. References: | |||||

RO5126766(CH5126766) Dilution Calculator

RO5126766(CH5126766) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1211 mL | 10.6054 mL | 21.2107 mL | 42.4214 mL | 53.0268 mL |
| 5 mM | 0.4242 mL | 2.1211 mL | 4.2421 mL | 8.4843 mL | 10.6054 mL |
| 10 mM | 0.2121 mL | 1.0605 mL | 2.1211 mL | 4.2421 mL | 5.3027 mL |
| 50 mM | 0.0424 mL | 0.2121 mL | 0.4242 mL | 0.8484 mL | 1.0605 mL |
| 100 mM | 0.0212 mL | 0.1061 mL | 0.2121 mL | 0.4242 mL | 0.5303 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
RO5126766 (CH5126766) is a first-in-class dual inhibitor of Raf/MEK [1].
The RAS/RAF/MEK/ERK signaling pathway is an important signal transduction system and participates in cell differentiation, movement, division and death. Activated Ras activates RAF kinase, which then phosphorylates and activates MEK (MEK1 and MEK2) [1]. The mutations in BRAF, RAS, and NF1 are associated with many human tumors [2].
RO5126766 (CH5126766) is a first-in-class dual Raf/MEK inhibitor. In cell-free kinase assays, CH5126766 effectively inhibited the phosphorylation of MEK1 protein by RAF and the activation of ERK2 protein by MEK1 with IC50 values of 0.0082-0.056 and 0.16 μM, respectively. In NCI-H460 (KRAS Q61H) human lung large cell carcinoma cell line, RO5126766 induced cell-cycle inhibitor p27Kip1 protein expression and caused G1 arrest. In HCT116 KRAS-mutant colorectal cancer cells, RO5126766 CH5126766 completely inhibited the phosphorylation of MEK and ERK [2].
In Japanese patients with advanced solid tumors, RO5126766 exhibited the maximum tolerable dose (MTD) of 2.25 mg/day once daily [1]. In a HCT116 (G13D KRAS) mouse xenograft model, RO5126766 (1.5 mg/kg) inhibited pERK and ERK signaling and exhibited ED50 value of 0.056 mg/kg [2].
References:
[1]. Honda K, Yamamoto N, Nokihara H, et al. Phase I and pharmacokinetic/pharmacodynamic study of RO5126766, a first-in-class dual Raf/MEK inhibitor, in Japanese patients with advanced solid tumors. Cancer Chemother Pharmacol, 2013, 72(3): 577-584.
[2]. Ishii N, Harada N, Joseph EW, et al. Enhanced inhibition of ERK signaling by a novel allosteric MEK inhibitor, CH5126766, that suppresses feedback reactivation of RAF activity. Cancer Res, 2013, 73(13): 4050-4060.
- LX-1031
Catalog No.:BCC1712
CAS No.:945976-76-1
- Senkyunolide I
Catalog No.:BCN6353
CAS No.:94596-28-8
- Senkyunolide H
Catalog No.:BCN6352
CAS No.:94596-27-7
- Parisyunnanoside B
Catalog No.:BCN2837
CAS No.:945865-37-2
- AA 29504
Catalog No.:BCC7829
CAS No.:945828-50-2
- XL019
Catalog No.:BCC2057
CAS No.:945755-56-6
- 7-O-ethyl-morroniside
Catalog No.:BCN3883
CAS No.:945721-10-8
- 6-Aldehydo-7-methoxyiso-ophiopogonanone B
Catalog No.:BCC8345
CAS No.:123941-06-0
- (R)-(+)-m-Nitrobiphenyline oxalate
Catalog No.:BCC7525
CAS No.:945618-97-3
- Pexmetinib (ARRY-614)
Catalog No.:BCC6509
CAS No.:945614-12-0
- AMG-900
Catalog No.:BCC2175
CAS No.:945595-80-2
- Levcromakalim
Catalog No.:BCC7039
CAS No.:94535-50-9
- Mulberrofuran K
Catalog No.:BCN7188
CAS No.:94617-36-4
- RN 1734
Catalog No.:BCC7770
CAS No.:946387-07-1
- LY2409881
Catalog No.:BCC5650
CAS No.:946518-60-1
- Hyperectine
Catalog No.:BCN3406
CAS No.:94656-46-9
- MNI 137
Catalog No.:BCC6156
CAS No.:946619-21-2
- Rhodiolgin; Gossypetin-7-O-α-rhamnopyranoside
Catalog No.:BCC8247
CAS No.:94696-39-6
- Gypenoside XLVI
Catalog No.:BCN3863
CAS No.:94705-70-1
- PF 429242
Catalog No.:BCC6009
CAS No.:947303-87-9
- Fmoc-Aib-OH
Catalog No.:BCC3149
CAS No.:94744-50-0
- Salmeterol xinafoate
Catalog No.:BCC1920
CAS No.:94749-08-3
- 7,3'-Dihydroxy-5'-methoxyisoflavone
Catalog No.:BCN3349
CAS No.:947611-61-2
- WWL 70
Catalog No.:BCC4011
CAS No.:947669-91-2
The dual RAF/MEK inhibitor CH5126766/RO5126766 may be a potential therapy for RAS-mutated tumor cells.[Pubmed:25422890]
PLoS One. 2014 Nov 25;9(11):e113217.
Although melanoma is the most aggressive skin cancer, recent advances in BRAF and/or MEK inhibitors against BRAF-mutated melanoma have improved survival rates. Despite these advances, a treatment strategy targeting NRAS-mutated melanoma has not yet been elucidated. We discovered CH5126766/RO5126766 as a potent and selective dual RAF/MEK inhibitor currently under early clinical trials. We examined the activity of CH5126766/RO5126766 in a panel of malignant tumor cell lines including melanoma with a BRAF or NRAS mutation. Eight cell lines including melanoma were assessed for their sensitivity to the BRAF, MEK, or RAF/MEK inhibitor using in vitro growth assays. CH5126766/RO5126766 induced G1 cell cycle arrest in two melanoma cell lines with the BRAF V600E or NRAS mutation. In these cells, the G1 cell cycle arrest was accompanied by up-regulation of the cyclin-dependent kinase inhibitor p27 and down-regulation of cyclinD1. CH5126766/RO5126766 was more effective at reducing colony formation than a MEK inhibitor in NRAS- or KRAS-mutated cells. In the RAS-mutated cells, CH5126766/RO5126766 suppressed the MEK reactivation caused by a MEK inhibitor. In addition, CH5126766/RO5126766 suppressed the tumor growth in SK-MEL-2 xenograft model. The present study indicates that CH5126766/RO5126766 is an attractive RAF/MEK inhibitor in RAS-mutated malignant tumor cells including melanoma.
[18 F]FDG-PET imaging is an early non-invasive pharmacodynamic biomarker for a first-in-class dual MEK/Raf inhibitor, RO5126766 (CH5126766), in preclinical xenograft models.[Pubmed:24041012]
EJNMMI Res. 2013 Sep 16;3(1):67.
BACKGROUND: Positron emission tomography (PET) with [2-18 F]-2-fluoro-2-deoxy-D-glucose ([18 F]FDG-PET) was acquired at multiple time-points a) to monitor the early response to RO5126766 (CH5126766) in xenograft models b) to evaluate non-invasive small animal [18 F]FDG-PET imaging as a biomarker for MEK inhibitors for translation into dose-finding studies in cancer patients and c) to explore the underlying mechanism related to FDG uptake in tumors treated with RO5126766. METHODS: [18 F]FDG uptake was studied in HCT116 (K-ras), COLO205 (B-raf) mutants and COLO320DM (wild type) xenografts from day 0 to 3 of RO5126766 treatment using a microPET Focus 120 and complemented with in vitro incubations, ex-vivo phosphor imaging and immunohistochemical (IHC) analyses. RESULTS: In the HCT116 (K-ras) and COLO205 (B-raf) mutant xenografts, significant decreases in [18 F]FDG uptake were detected in vivo on day 1 with 0.3 mg/kg and ex vivo on day 3 with 0.1 mg/kg RO5126766. [18 F]FDG changes correlated with decreases in tumor cells proliferation (Ki-67) and with changes in expression levels of GLUT1. No effects were observed in drug resistant COLO320DM cells. The cellular fractionation and Western blotting analyses suggested that the change of [18 F]FDG uptake associated with RO5126766 is due to translocation of GLUT1 from membrane to cytosol, similar to the results reported in the literature with EGFR tyrosine kinase inhibitors, which also target the MAPK pathway. CONCLUSIONS: RO5126766 inhibition resulted in a rapid time - and dose - dependent decline in [18 F]FDG uptake in both mutant xenografts. These results strongly resemble the clinical observations obtained with MEK/Raf inhibitors support the use of preclinical [18 F]FDG-PET as a translational tool for decision support in preclinical and early clinical development of MEK inhibitors.


