LY2409881CAS# 946518-60-1 |
- CX-5461
Catalog No.:BCC3700
CAS No.:1138549-36-6
- Gemcitabine HCl
Catalog No.:BCC1076
CAS No.:122111-03-9
- Fludarabine Phosphate (Fludara)
Catalog No.:BCC3681
CAS No.:75607-67-9
- Bleomycin Sulfate
Catalog No.:BCC3694
CAS No.:9041-93-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 946518-60-1 | SDF | Download SDF |
| PubChem ID | 68856073 | Appearance | Powder |
| Formula | C24H32Cl4N6OS | M.Wt | 594.43 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in DMSO | ||
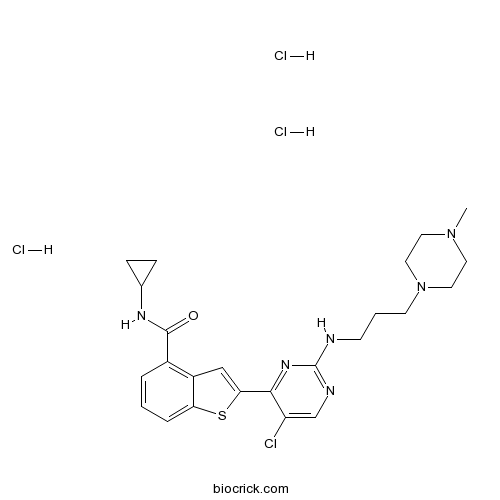
| Chemical Name | 2-[5-chloro-2-[3-(4-methylpiperazin-1-yl)propylamino]pyrimidin-4-yl]-N-cyclopropyl-1-benzothiophene-4-carboxamide;trihydrochloride | ||
| SMILES | CN1CCN(CC1)CCCNC2=NC=C(C(=N2)C3=CC4=C(C=CC=C4S3)C(=O)NC5CC5)Cl.Cl.Cl.Cl | ||
| Standard InChIKey | IEXCRLAGBWKVPW-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C24H29ClN6OS.3ClH/c1-30-10-12-31(13-11-30)9-3-8-26-24-27-15-19(25)22(29-24)21-14-18-17(4-2-5-20(18)33-21)23(32)28-16-6-7-16;;;/h2,4-5,14-16H,3,6-13H2,1H3,(H,28,32)(H,26,27,29);3*1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

LY2409881 Dilution Calculator

LY2409881 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.6823 mL | 8.4114 mL | 16.8228 mL | 33.6457 mL | 42.0571 mL |
| 5 mM | 0.3365 mL | 1.6823 mL | 3.3646 mL | 6.7291 mL | 8.4114 mL |
| 10 mM | 0.1682 mL | 0.8411 mL | 1.6823 mL | 3.3646 mL | 4.2057 mL |
| 50 mM | 0.0336 mL | 0.1682 mL | 0.3365 mL | 0.6729 mL | 0.8411 mL |
| 100 mM | 0.0168 mL | 0.0841 mL | 0.1682 mL | 0.3365 mL | 0.4206 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
LY2409881 is a potent and selective IKK2 inhibitor with IC50 of 30 nM, >10-fold selectivity over IKK1 and other common kinases.
- RN 1734
Catalog No.:BCC7770
CAS No.:946387-07-1
- Mulberrofuran K
Catalog No.:BCN7188
CAS No.:94617-36-4
- RO5126766(CH5126766)
Catalog No.:BCC6374
CAS No.:946128-88-7
- LX-1031
Catalog No.:BCC1712
CAS No.:945976-76-1
- Senkyunolide I
Catalog No.:BCN6353
CAS No.:94596-28-8
- Senkyunolide H
Catalog No.:BCN6352
CAS No.:94596-27-7
- Parisyunnanoside B
Catalog No.:BCN2837
CAS No.:945865-37-2
- AA 29504
Catalog No.:BCC7829
CAS No.:945828-50-2
- XL019
Catalog No.:BCC2057
CAS No.:945755-56-6
- 7-O-ethyl-morroniside
Catalog No.:BCN3883
CAS No.:945721-10-8
- 6-Aldehydo-7-methoxyiso-ophiopogonanone B
Catalog No.:BCC8345
CAS No.:123941-06-0
- (R)-(+)-m-Nitrobiphenyline oxalate
Catalog No.:BCC7525
CAS No.:945618-97-3
- Hyperectine
Catalog No.:BCN3406
CAS No.:94656-46-9
- MNI 137
Catalog No.:BCC6156
CAS No.:946619-21-2
- Rhodiolgin; Gossypetin-7-O-α-rhamnopyranoside
Catalog No.:BCC8247
CAS No.:94696-39-6
- Gypenoside XLVI
Catalog No.:BCN3863
CAS No.:94705-70-1
- PF 429242
Catalog No.:BCC6009
CAS No.:947303-87-9
- Fmoc-Aib-OH
Catalog No.:BCC3149
CAS No.:94744-50-0
- Salmeterol xinafoate
Catalog No.:BCC1920
CAS No.:94749-08-3
- 7,3'-Dihydroxy-5'-methoxyisoflavone
Catalog No.:BCN3349
CAS No.:947611-61-2
- WWL 70
Catalog No.:BCC4011
CAS No.:947669-91-2
- TCFH
Catalog No.:BCC2824
CAS No.:94790-35-9
- HBTU
Catalog No.:BCC2814
CAS No.:94790-37-1
- ML365
Catalog No.:BCC8063
CAS No.:947914-18-3
The novel IKK2 inhibitor LY2409881 potently synergizes with histone deacetylase inhibitors in preclinical models of lymphoma through the downregulation of NF-kappaB.[Pubmed:25355930]
Clin Cancer Res. 2015 Jan 1;21(1):134-45.
PURPOSE: To evaluate the pharmacologic activity of a novel inhibitor of IkappaB kinase beta (IKK2), LY2409881, in preclinical models of B- and T-cell lymphoma, as a single agent and in combination with histone deacetylase (HDAC) inhibitors. EXPERIMENTAL DESIGN: The in vitro activity of LY2409881 was determined using an ATP-based growth inhibition assay and flow cytometric assay of apoptosis in lymphoma cell lines. The in vivo activity of LY2409881 was determined using SCID-beige xenograft mouse model. The mechanism of action was determined using immunoblotting, immuofluorescence, and electrophoretic mobility shift assay. Synergy of LY2409881 with other drugs active in lymphoma was determined by calculating relative risk ratio (RRR) and combination index (CI). RESULTS: LY2409881 inhibited constitutively activated NF-kappaB, and caused concentration- and time-dependent growth inhibition and apoptosis in lymphoma cells. In models of diffuse large B-cell lymphoma (DLBCL), the cytotoxicity of LY2409881 correlated with the overall activation status of NF-kappaB, but not simply in a pattern predicted by the cell-of-origin classification of these cell lines. LY2409881 was safe to mice at three dose levels, 50, 100, and 200 mg/kg, all of which caused significant inhibition of tumor growth. LY2409881 suppressed the activity of the NF-kappaB subunit p65 in lymphoma cells treated by the HDAC inhibitor romidepsin, underlying a potential mechanism of the marked synergy observed of these two drugs. CONCLUSION: Collectively, these data strongly suggest that targeting the NF-kappaB pathway in combination with romidepsin could represent a novel and potent regimen for the treatment of B- and T-cell lymphoma.


