HBTUPeptide coupling reagent CAS# 94790-37-1 |
- PD128907 HCl
Catalog No.:BCC4469
CAS No.:112960-16-4
- Pramipexole 2HCl Monohydrate
Catalog No.:BCC4466
CAS No.:191217-81-9
- Clozapine
Catalog No.:BCC5037
CAS No.:5786-21-0
- LY404039
Catalog No.:BCC4592
CAS No.:635318-11-5
- Pergolide mesylate
Catalog No.:BCC4464
CAS No.:66104-23-2
- Brexpiprazole
Catalog No.:BCC4118
CAS No.:913611-97-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 94790-37-1 | SDF | Download SDF |
| PubChem ID | 130500 | Appearance | Powder |
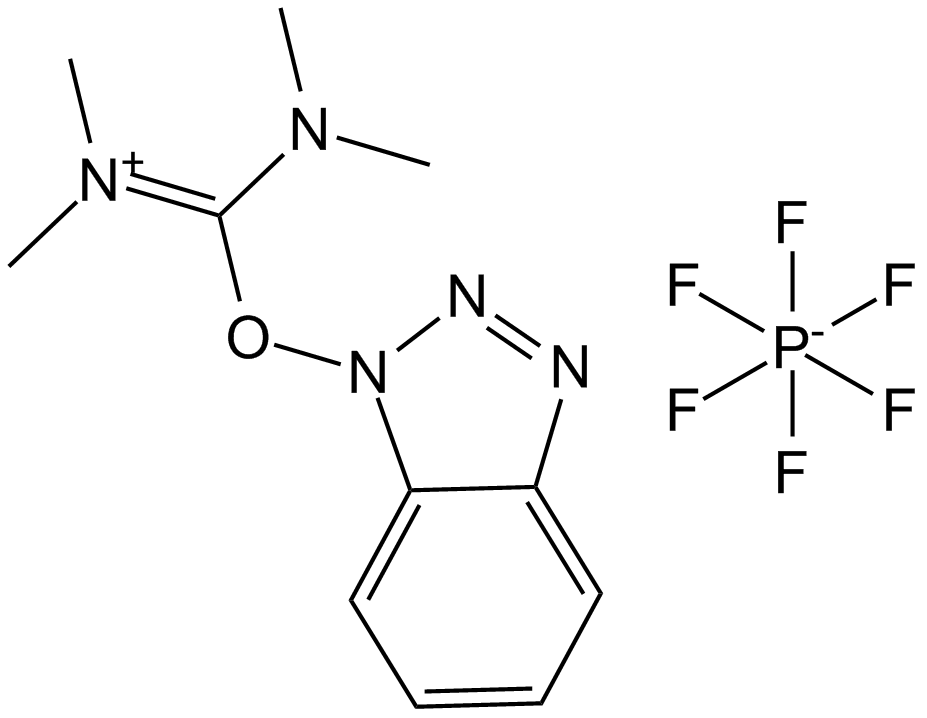
| Formula | C11H16F6N5OP | M.Wt | 379.3 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | >37.9mg/mL in DMSO | ||
| Chemical Name | [benzotriazol-1-yloxy(dimethylamino)methylidene]-dimethylazanium | ||
| SMILES | CN(C)C(=[N+](C)C)ON1C2=CC=CC=C2N=N1 | ||
| Standard InChIKey | CLZISMQKJZCZDN-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C11H16N5O/c1-14(2)11(15(3)4)17-16-10-8-6-5-7-9(10)12-13-16/h5-8H,1-4H3/q+1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | HBTU is a coupling reagent for peptide synthesis. |

HBTU Dilution Calculator

HBTU Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
IC50: Not available.
HBTU (2-(1H-benzotriazol-1-yl)-1,1,3,3-tetramethyluronium hexauorophosphate), a coupling reagent commonly used in solid phase peptide synthesis. After being introduced in 1978, this agent shows popularity in chemistry and industry use due to its mild activating properties. Moreover, it also shows resistance against racemization. Low tendency for racemization is a key requirement for peptide synthesis. Especially for solid phase peptide synthesis, quantitative yields with short reaction times are crucial in order to make the synthesis of large peptides feasible. [1]
In vitro: Peptide synthesis relied mostly on efficient and safe coupling reagents. HBTU was proved to transform carboxylic acids into azides efficiently and practically. The process might be applied to a wide range of carboxylic acids including N-protected amino acids. In addition, HBTU was of great value in one-pot synthesis of dipeptidyl urea esters, ureas, and carbamates from acids. The advantages of HBTU included the following points: 1) Non-explosive and therefore more suitable for solution/solid phase peptide synthesis. 2) High solubility and stability in classical solvents. 3) Feasible for colorimetric reaction monitoring. [2]
In vivo: So far, no in vivo data has been reported.
Clinical trial: So far, no clinical trial has been conducted.
References:
[1]Adam S. HBTU: a mild activating ageiw of muramic acid. Bioorg Med Chem Lett. 1992 Mar; 2(6): 571-4.
[2]Knorr R, Trzeciak A, Bannwarth W and Gillessen D. New coupling reagents in peptide chemistry. Tetrahedron Lett. 1989; 30(15): 1927-30.
- TCFH
Catalog No.:BCC2824
CAS No.:94790-35-9
- WWL 70
Catalog No.:BCC4011
CAS No.:947669-91-2
- 7,3'-Dihydroxy-5'-methoxyisoflavone
Catalog No.:BCN3349
CAS No.:947611-61-2
- Salmeterol xinafoate
Catalog No.:BCC1920
CAS No.:94749-08-3
- Fmoc-Aib-OH
Catalog No.:BCC3149
CAS No.:94744-50-0
- PF 429242
Catalog No.:BCC6009
CAS No.:947303-87-9
- Gypenoside XLVI
Catalog No.:BCN3863
CAS No.:94705-70-1
- Rhodiolgin; Gossypetin-7-O-α-rhamnopyranoside
Catalog No.:BCC8247
CAS No.:94696-39-6
- MNI 137
Catalog No.:BCC6156
CAS No.:946619-21-2
- Hyperectine
Catalog No.:BCN3406
CAS No.:94656-46-9
- LY2409881
Catalog No.:BCC5650
CAS No.:946518-60-1
- RN 1734
Catalog No.:BCC7770
CAS No.:946387-07-1
- ML365
Catalog No.:BCC8063
CAS No.:947914-18-3
- 3-pyr-Cytisine
Catalog No.:BCC6118
CAS No.:948027-43-8
- Bruceantinol A
Catalog No.:BCN8003
CAS No.:948038-36-6
- Bruceine J
Catalog No.:BCN8001
CAS No.:948038-38-8
- 20(R)-Notoginsenoside R2
Catalog No.:BCN3864
CAS No.:948046-15-9
- Glycycoumarin
Catalog No.:BCN7685
CAS No.:94805-82-0
- Isolicoflavonol
Catalog No.:BCN4554
CAS No.:94805-83-1
- Tie2 kinase inhibitor
Catalog No.:BCC2561
CAS No.:948557-43-5
- Acuminatanol
Catalog No.:BCN6866
CAS No.:948884-38-6
- 2beta-(Isobutyryloxy)florilenalin
Catalog No.:BCN7976
CAS No.:94898-78-9
- Z-Gly-NH2
Catalog No.:BCC2769
CAS No.:949-90-6
- H-Phe(4-NO2)-OH
Catalog No.:BCC3293
CAS No.:949-99-5
HBTU mediated 1-hydroxybenzotriazole (HOBt) conjugate addition: synthesis and stereochemical analysis of beta-benzotriazole N-oxide substituted gamma-amino acids and hybrid peptides.[Pubmed:25228027]
Org Biomol Chem. 2014 Nov 14;12(42):8462-72.
HBTU is a standard coupling agent commonly used for the activation of free carboxylic acids during the solution and solid phase peptide synthesis. 1-Hydroxybenzotriazole (HOBt) plays a significant role in reducing the racemization during peptide synthesis; hence it is regularly used as a coupling additive. Here, we are reporting the mild and facile conjugate addition of HOBt to E-vinylogous gamma-amino acids mediated by the HBTU. The reaction is moderately diastereoselective and novel beta-benzotriazole N-oxide (beta-BtO) substituted gamma-amino acids were isolated in moderate to good yields. The single crystal analysis of methyl esters of major (anti) and minor (syn) conjugate addition products infers the formation of exclusively N-alkylated benzotriazole N-oxides instead of O-alkylation of HOBt. In addition, we showed the utilization of beta-BtO substituted gamma-amino acids in peptide synthesis and studied their conformations in single crystals.
New and simple synthesis of acid azides, ureas and carbamates from carboxylic acids: application of peptide coupling agents EDC and HBTU.[Pubmed:20135041]
Org Biomol Chem. 2010 Feb 21;8(4):835-40.
Conversion of carboxylic acids into acid azides using peptide coupling agents, EDC and HBTU is described. The procedure is efficient, practical and applicable to a diverse range of carboxylic acids including N-protected amino acids. Using the same reagents, one-pot synthesis of ureas, dipeptidyl urea esters and carbamates from acids has also been achieved.
Anaphylaxis and allergic contact urticaria from occupational airborne exposure to HBTU.[Pubmed:16861336]
Occup Med (Lond). 2006 Sep;56(6):430-3.
We describe a case of anaphylaxis and allergic contact urticaria from occupational airborne exposure to HBTU (o-(benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate), which is a chemical used widely for solid and solution-phase peptide synthesis. Previously, the use of this chemical has been associated with occupational asthma, allergic contact urticaria and allergic contact dermatitis in individual cases, but not with anaphylaxis. Our diagnoses were based on the clinical symptoms, positive skin prick test (SPT) and positive skin provocation test to HBTU. The positive SPT indicates that the anaphylaxis reaction was IgE-mediated. We recommend that in the handling of HBTU, appropriate safety measures should be compulsory, and if work-related symptoms develop, the possibility of anaphylaxis should be considered in advising on appropriate work tasks.


