Sorbic acid, 1-p-tolylhydrazideCAS# 802048-02-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 802048-02-8 | SDF | Download SDF |
| PubChem ID | 131859799 | Appearance | Powder |
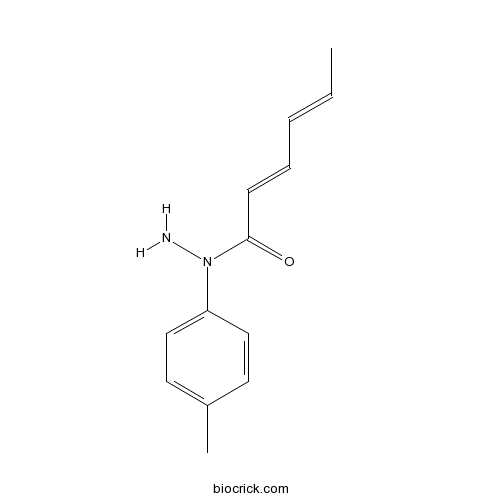
| Formula | C13H16N2O | M.Wt | 216.28 |
| Type of Compound | Miscellaneous | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | N-(4-methylphenyl)hexa-2,4-dienehydrazide | ||
| SMILES | CC=CC=CC(=O)N(C1=CC=C(C=C1)C)N | ||
| Standard InChIKey | SFUOGWMMHCFCBL-UHFFFAOYSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Sorbic acid, 1-p-tolylhydrazide Dilution Calculator

Sorbic acid, 1-p-tolylhydrazide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.6236 mL | 23.1182 mL | 46.2364 mL | 92.4727 mL | 115.5909 mL |
| 5 mM | 0.9247 mL | 4.6236 mL | 9.2473 mL | 18.4945 mL | 23.1182 mL |
| 10 mM | 0.4624 mL | 2.3118 mL | 4.6236 mL | 9.2473 mL | 11.5591 mL |
| 50 mM | 0.0925 mL | 0.4624 mL | 0.9247 mL | 1.8495 mL | 2.3118 mL |
| 100 mM | 0.0462 mL | 0.2312 mL | 0.4624 mL | 0.9247 mL | 1.1559 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Helicid
Catalog No.:BCN1056
CAS No.:80154-34-3
- Methyl demethoxycarbonylchanofruticosinate
Catalog No.:BCN1348
CAS No.:80151-89-9
- Tussilagine
Catalog No.:BCN1984
CAS No.:80151-77-5
- L-Alanosine
Catalog No.:BCN7253
CAS No.:5854-93-3
- GSK256066
Catalog No.:BCC2285
CAS No.:801312-28-7
- YC 170
Catalog No.:BCC1212
CAS No.:59946-73-5
- H-D-Phe(3-Cl)-OH
Catalog No.:BCC3167
CAS No.:80126-52-9
- H-Phe(3-Cl)-OH
Catalog No.:BCC3168
CAS No.:80126-51-8
- H-D-Phe(2-Cl)-OH
Catalog No.:BCC3166
CAS No.:80126-50-7
- Boc-D-Phe(3-Cl)-OH
Catalog No.:BCC3169
CAS No.:80102-25-6
- Agatharesinol acetonide
Catalog No.:BCN4574
CAS No.:800389-33-7
- Soybean phospholipid
Catalog No.:BCN3888
CAS No.:8002-43-5
- Roxithromycin
Catalog No.:BCC4842
CAS No.:80214-83-1
- Glochidionionol C
Catalog No.:BCC2641
CAS No.:
- Rosmanol
Catalog No.:BCN8425
CAS No.:80225-53-2
- 2'-O-Galloylquercitrin
Catalog No.:BCN8225
CAS No.:80229-08-9
- Casanthranol
Catalog No.:BCC3746
CAS No.:8024-48-4
- Spiramycin
Catalog No.:BCC4724
CAS No.:8025-81-8
- Nafamostat hydrochloride
Catalog No.:BCC4188
CAS No.:80251-32-7
- PHA-848125
Catalog No.:BCC3839
CAS No.:802539-81-7
- Artemisinic acid
Catalog No.:BCN4336
CAS No.:80286-58-4
- AZD1981
Catalog No.:BCC4506
CAS No.:802904-66-1
- RG7090
Catalog No.:BCC5499
CAS No.:802906-73-6
- Dehydro-δ-tocopherol
Catalog No.:BCN4573
CAS No.:802909-72-4
Development of a LC-MS/MS method for the simultaneous determination of sorbic acid, natamycin and tylosin in Dulce de leche.[Pubmed:27283692]
Food Chem. 2016 Nov 15;211:748-56.
A simple extraction, rapid routine method for the simultaneous determination of sorbic acid, natamycin and tylosin in Dulce de leche, a traditional South American product, by liquid chromatography-tandem mass spectrometry has been developed and fully validated. The limits of detection were set to 24.41mgkg(-1) (sorbic acid), 0.10mgkg(-1) (natamycin) and 2mugkg(-1) (tylosin). Recoveries ranged from 95% to 110%. Proportionally, internal standardization was more efficient than external standard, resulting in a smaller measurement of uncertainty. In total, 35 commercial samples from Brazil, Argentina and Uruguay have been assessed. The proposed method was tested on other dairy desserts, demonstrating to be versatile. Although tylosin was not detected in any sample, a high rate of non-compliance was found, with 67.39% of samples above the maximum allowed for sorbic acid and a maximum concentration of 2105.36+/-178.60mgkg(-1). In two samples, natamycin was irregularly found.
In-capillary derivatization with o-phthalaldehyde in the presence of 3-mercaptopropionic acid for the simultaneous determination of monosodium glutamate, benzoic acid, and sorbic acid in food samples via capillary electrophoresis with ultraviolet detection.[Pubmed:27156753]
J Chromatogr A. 2016 Jun 3;1449:156-65.
For the rapid simultaneous determination of monosodium glutamate (MSG), benzoic acid (BA), and sorbic acid (SA) in canned food and other processed food samples, we developed a method that combines in-capillary derivatization with separation by capillary electrophoresis. This method employs the rapid derivatization of MSG with o-phthalaldehyde (OPA) in the presence of 3-mercaptopropionic acid (3-MPA) and enables the detection of the resulting OPA-MSG derivative and of non-derivatized BA and SA at 230nm. The composition of the background electrolyte and the parameters of derivatization and separation are as follows: 25mM borax containing 5mM OPA and 6mM 3-MPA, separation voltage 25mV, injection at 30mbar for 20s, and column temperature 25 degrees C. Because of the high reaction rate and suitably adapted effective electrophoretic mobilities, band broadening due to the derivatization reaction at the start of the separation process is kept to a minimum. The optimized method is validated with respect to LOD, LOQ, linearity, recovery, and precision. This method can be applied to real samples such as soy, fish, oyster and sweet and sour chili sauces after application of appropriate clean-up steps. Mechanisms of zone broadening and zone focusing are discussed showing the validity of the employed theoretical approach regarding the dependence of the peak shape for OPA-MSG on the concentration of MSG in the sample.
Quantifying the effect of sorbic acid, heat and combination of both on germination and outgrowth of Bacillus subtilis spores at single cell resolution.[Pubmed:26338121]
Food Microbiol. 2015 Dec;52:88-96.
Bacillus subtilis spores are a problem for the food industry as they are able to survive preservation processes. The spores often reside in food products, where their inherent protection against various stress treatments causes food spoilage. Sorbic acid is widely used as a weak acid preservative in the food industry. Its effect on spore germination and outgrowth in a combined, 'hurdle', preservation setting has gained limited attention. Therefore, the effects of mild sorbic acid (3 mM), heat-treatment (85 degrees C for 10 min) and a combination of both mild stresses on germination and outgrowth of B. subtilis 1A700 spores were analysed at single spore level. The heat-treatment of the spore population resulted in a germination efficiency of 46.8% and an outgrowth efficiency of 32.9%. In the presence of sorbic acid (3 mM), the germination and outgrowth efficiency was 93.3% and 80.4% respectively whereas the combined heat and sorbic acid stress led to germination and outgrowth efficiencies of 52.7% and 27.0% respectively. The heat treatment clearly primarily affected the germination process, while sorbic acid affected the outgrowth and generation time. In addition a new 'burst' time-point was defined as the time-point at which the spore coat visibly breaks and/or is shed. The combined stresses had a synergistic effect on the time of the end of germination to the burst time-point, increasing both the mean and its variation more than either of the single stresses did.
Survival of the functional yeast Kluyveromyces marxianus B0399 in fermented milk with added sorbic acid.[Pubmed:26547644]
J Dairy Sci. 2016 Jan;99(1):120-9.
In this study, the survival of the functional yeast Kluyveromyces marxianus B0399 in an industrially produced fermented milk was evaluated. In particular, the yeast viability was assessed throughout the entire shelf-life of the product (30 d) to ensure the presence of the effective yeast dose (20 million viable cells for each serving of 125 g) while avoiding, by sorbic acid addition, yeast growth, which could affect product quality and stability. To find the best combination of yeast and sorbic acid concentration, 13 different combinations were tested, and then 2 of them were chosen for industrial production. In production at lower concentrations (30 million viable cells, 150 mg/kg of sorbic acid) the effective dose was maintained only at 4 and 6 degrees C, whereas at higher dosages (70 million viable cells, 250 mg/kg of sorbic acid) the effect of temperature was less evident. In all the trials, the concentration of sorbic acid was not affected by microbial metabolism and remained stable throughout the entire shelf-life.


