SyringetinCAS# 4423-37-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 4423-37-4 | SDF | Download SDF |
| PubChem ID | 5281953 | Appearance | Powder |
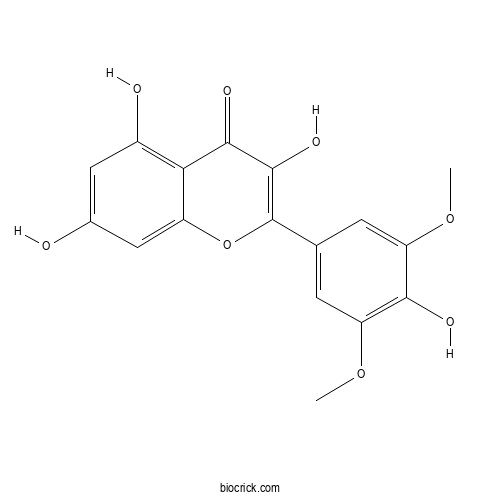
| Formula | C17H14O8 | M.Wt | 346.3 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 3,5,7-trihydroxy-2-(4-hydroxy-3,5-dimethoxyphenyl)chromen-4-one | ||
| SMILES | COC1=CC(=CC(=C1O)OC)C2=C(C(=O)C3=C(C=C(C=C3O2)O)O)O | ||
| Standard InChIKey | UZMAPBJVXOGOFT-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C17H14O8/c1-23-11-3-7(4-12(24-2)14(11)20)17-16(22)15(21)13-9(19)5-8(18)6-10(13)25-17/h3-6,18-20,22H,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Syringetin prevents and treats bone metastasis in patients with lung cancer, it can directly inhibits osteoclastogenesis and reverses lung adenocarcinoma‑mediated osteoclastogenesis. Syringetin can increase BMP-2 synthesis, and subsequently activate SMAD1/5/8 and ERK1/2, and this effect may contribute to its action on the induction of osteoblast maturation and differentiation, followed by an increase of bone mass. | |||||

Syringetin Dilution Calculator

Syringetin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8877 mL | 14.4383 mL | 28.8767 mL | 57.7534 mL | 72.1917 mL |
| 5 mM | 0.5775 mL | 2.8877 mL | 5.7753 mL | 11.5507 mL | 14.4383 mL |
| 10 mM | 0.2888 mL | 1.4438 mL | 2.8877 mL | 5.7753 mL | 7.2192 mL |
| 50 mM | 0.0578 mL | 0.2888 mL | 0.5775 mL | 1.1551 mL | 1.4438 mL |
| 100 mM | 0.0289 mL | 0.1444 mL | 0.2888 mL | 0.5775 mL | 0.7219 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Norharman
Catalog No.:BCN9874
CAS No.:244-63-3
- 2-(4-Hydroxybenzal)acetophenone
Catalog No.:BCN9873
CAS No.:20426-12-4
- Condurango glycoside A
Catalog No.:BCN9872
CAS No.:11051-90-4
- Lappaconine
Catalog No.:BCN9871
CAS No.:23943-93-3
- Cannabiscitrin
Catalog No.:BCN9870
CAS No.:520-14-9
- Datiscin
Catalog No.:BCN9869
CAS No.:16310-92-2
- 15-Methoxy-16-oxo-15,16H-strictic acid
Catalog No.:BCN9868
CAS No.:1356388-38-9
- 1,2-Dimethoxybenzene
Catalog No.:BCN9867
CAS No.:91-16-7
- Tropic acid
Catalog No.:BCN9866
CAS No.:529-64-6
- 4-(4-Methoxyphenyl)-2-butanone
Catalog No.:BCN9865
CAS No.:104-20-1
- ICG-001
Catalog No.:BCN9864
CAS No.:780757-88-2
- 3,29-O-Dibenzoyloxykarounidiol
Catalog No.:BCN9863
CAS No.:389122-01-4
- 4-Hydroxyisophthalic acid
Catalog No.:BCN9876
CAS No.:636-46-4
- Lyciumamide B
Catalog No.:BCN9877
CAS No.:1647111-41-8
- Tricaprin
Catalog No.:BCN9878
CAS No.:621-71-6
- Dihydroquinine
Catalog No.:BCN9879
CAS No.:522-66-7
- Cymarin
Catalog No.:BCN9880
CAS No.:508-77-0
- 5,7-Dihydroxy 3,3',4',5',6,8-hexamethoxyflavone
Catalog No.:BCN9881
CAS No.:96887-18-2
- gamma-Decalactone
Catalog No.:BCN9882
CAS No.:706-14-9
- 2-(2-Hydroxybenzal)acetophenone
Catalog No.:BCN9883
CAS No.:644-78-0
- (1S,2S,5S)-(-)-Myrtanol
Catalog No.:BCN9884
CAS No.:53369-17-8
- 6-Methoxyflavanone
Catalog No.:BCN9885
CAS No.:3034-04-6
- 15-Deoxypulic acid
Catalog No.:BCN9886
CAS No.:95523-05-0
- 4-Oxadocosane-1,2-diol
Catalog No.:BCN9887
CAS No.:544-62-7
Flavonoids as BACE1 inhibitors: QSAR modelling, screening and in vitro evaluation.[Pubmed:33010267]
Int J Biol Macromol. 2020 Dec 15;165(Pt A):1323-1330.
Alzheimer's disease (AD) is marked by the presence of amyloid plaques, neurofibrillary tangles, oxidatively damaged neuronal macromolecules and redox sensitive ions. Reduction of amyloid plaques and oxidative stress emerge as a convincing treatment strategy. Plaque reduction is achieved by inhibition of BACE1, the rate limiting enzyme generating the prime constituent of plaques, Abeta, through proteolysis of the amyloid precursor protein. Here, we report a QSAR model with five descriptors, developed to screen natural compounds as potent BACE1 inhibitors. Seven compounds out of which five flavonols namely isorhamnetin, Syringetin, galangin, tamarixetin, rhamnetin and two flavanonols namely dihydromyricetin, taxifolin were screened. The ability of these compounds were validated using the BACE1 activity assay. The antioxidant property were estimated by the DPPH and ABTS assay. Although inhibition assay implied Syringetin to be a promising BACE1 inhibitor, its poor antioxidant activity leaves it less effective as a multitarget ligand. Exhibiting moderate dual ability, isorhamnetin and taxifolin qualified as multi-target scaffolds for AD therapeutics. Our study reveals the importance of 4'-OH in the B ring of flavonols and the lack of any effect of 5'-OH in flavanonols for BACE1 inhibition. In case of antioxidant activity favourable association of 3'-O-methylation derivatives was observed in flavonols.
Phenolics profiling by HPLC-DAD-ESI-MS(n) aided by principal component analysis to classify Rabbiteye and Highbush blueberries.[Pubmed:32916406]
Food Chem. 2021 Mar 15;340:127958.
Although blueberries are widely studied, little information exists on their composition and content of flavonol glycosides. Most studies identify only a few flavonols in blueberries due to separation and identification issues. In the present study, we identified 44 flavonols and chlorogenic acid in 30 samples of Highbush and Rabbiteye blueberry, using HPLC-DAD-ESI-MS(n). Highbush group fruits presented mainly quercetin-3-galactoside in their composition, while Rabbiteye group fruits exhibited higher levels of quercetin-3-rhamnoside and quercetin-3-glucuronide. Among the identified flavonols, 8 acylates (acetyl and hydroxymethylglutaroyl) were found, of which quercetin-3-O-[4''-(3-hydroxy-3-methylglutaroyl)]-alpha-rhamnoside was found for the first time in blueberries. This compound is exclusive to the cultivars Florida and Powderblue, where it is present in high quantities. Glucuronides of Syringetin and laricitrin, and rhamnosyl-galactosides of myricetin, quercetin and isorhamnetin were also found for the first time in blueberries. The Principal Component Analysis showed that blueberry groups can be distinguished based on their phenolic compound profile.
Phytochemical characterization of different yarrow species (Achillea sp.) and investigations into their antimicrobial activity.[Pubmed:32897872]
Z Naturforsch C J Biosci. 2020 Sep 8. pii: /j/znc.ahead-of-print/znc-2020-0149/znc-2020-0149.xml.
Various Achillea species are rich in bioactive compounds and are important medicinal plants in phytotherapy. In the present study, Achillea millefolium L., Achillea moschata Wulfen, and Achillea atrata L. were compared with respect to their phenolic profile and antibacterial activity against gram-positive bacteria strains (Staphylococcus, Propionibacterium). Particular focus was given to A. atrata, which has hardly been studied so far. Based on the metabolite profile, A. atrata exhibited more similarities to A. moschata than to A. millefolium. The former two only differed in the occurrence of four compounds. The flavonols Syringetin-3-O-glucoside and mearnsetin-hexoside, not reported for an Achillea species before, have been detected in A. atrata and A. moschata. All Achillea species reduced growth of the tested bacteria. A. atrata demonstrated highest activity against Propionibacterium acnes and Staphylococcus epidermidis, both being involved in the pathogenesis of acne vulgaris. Furthermore, A. atrata has a pronounced anti-methicillin-resistant Staphylococcus aureus potential. Bioassay-guided fractionation revealed that only the most polar fraction of A. moschata displayed antimicrobial activity, which was attributed to phenolics such as apigenin, centaureidin, and nevadensin, being present in high amounts in A. atrata. Thus, this alpine species shows promising antimicrobial activity and might be a potential source for developing novel dermal/topical drugs.
New acylated flavonols identified in Vitis vinifera grapes and wines.[Pubmed:30131163]
Food Res Int. 2018 Oct;112:98-107.
Flavonols are a class of polyphenol compounds whose importance for wine quality has increased as their structures and properties have become better understood. Here, the acetylated and p-coumaroylated derivatives of the flavonol 3-O-glucosides of isorhamnetin, laricitrin and Syringetin have been identified for the first time in Vitis vinifera grape skins and wines. First, the MS(2) fragmentation patterns of the new flavonol derivatives showed a main signal attributable to the expected flavonol aglycone. In the p-coumaroylated derivatives, the signal corresponding to the intermediate loss of the phenolic acid was also observed. The structures of the aglycones were confirmed by their respective MS(3) experiments that matched with those obtained from authentic standards of the aglycones. In addition, the fragmentation signals corresponding to the aglycone radical ions generated through homolytic cleavage assisted identification, and could support future studies of flavonoid compounds by ESI-MS. Using an HPLC-ESI-Q-ToF system, the observed m/z values of the compounds being studied were successfully matched with the expected formula. Surprisingly, just the minority methoxylated flavonol glucosides presented acylation, suggesting a high substrate specificity of the acyltransferases implicated in their synthesis. These findings show higher diversity of grape and wine flavonols. Additional studies and isolation strategies need to be followed to further characterize these metabolites as to test their presence in other grape varieties and it wines.
LC-QTOF characterization of non-anthocyanic flavonoids in four Tunisian fig varieties.[Pubmed:29859515]
J Mass Spectrom. 2018 Sep;53(9):817-823.
Flavonoids are compounds characterized by antioxidant activity, and their intake in the human diet is considered useful for health and nutrition. Non-anthocyanic flavonoids in 4 different types of Tunisian figs belonging to the smyrna-type Ficus carica varieties known as Kholi, Tchich Asal, Himri, and Bidhi were studied by liquid chromatography/high-resolution mass spectrometry UHPLC-QqTOF. Twenty-two compounds belonging to the classes of flavanones (naringenin and eriodictyol), flavones (3 apigenin and 5 luteolin derivatives), and flavonols (2 kaempferol and 7 quercetin derivatives) were identified. Three O-methoxy flavonols (tamarixetin, Syringetin, and isorhamnetin-3-O-glucoside) were found in figs for the first time. Total content of non-anthocyanic flavonoids found in dark varieties (between 410 and 830 mg/kg) show that these F. carica are fruits qualitatively and quantitatively rich of dietary polyphenols.
Glycosylation and Methylation of Quercetin and Myricetin by Cultured Cells of Phytolacca americana.[Pubmed:30520587]
Nat Prod Commun. 2017 Apr;12(4):523-524.
The glycosylation and methylation of quercetin by cultured plant cells of Phytolacca americana gave quercetin 3-Omicron-beta-D-glucoside and isorharnnetin 3-Omicron-beta-D- glucoside. Myricetin was glycosylated and methylated to Syringetin 3-Omicron-beta-D-glucoside by cultured P. americana cells.
Potentiality of syringetin for preferential radiosensitization to cancer cells.[Pubmed:27707083]
Int J Radiat Biol. 2017 Mar;93(3):286-294.
PURPOSE: To examine the enhancing effects of Syringetin on the radiosensitivity of normal and cancer cells, and the related mechanism. MATERIALS AND METHODS: We used normal human lung and mouse fibroblasts as well as human lung and mouse cancer cells derived from the above normal fibroblasts. Cell radiosensitivity was measured using a colony formation assay. Apoptosis was analyzed with DAPI staining and Western blots. DNA lesions were analyzed with gammaH2AX immunofluorescent staining. RESULTS: The colony formation assay showed that Syringetin enhanced radiosensitivity more effectively in cancer cells (H1299 and C3H/MCA clone 15) compared with normal cells (HFL-III and C3H/10T1/2). The radiosensitizing effect of Syringetin was observed in mutated p53 and wild-type p53-transfected H1299 cells regardless of p53 status. Apoptosis was more frequently observed in X-ray-irradiated H1299 cells combined with Syringetin compared with X-ray-only-treated cells. Enhanced apoptosis by Syringetin was not observed in HFL-III cells. Western blot analysis showed that X-ray-induced Caspase-3 activation was enhanced by Syringetin in H1299 cells. The number of X-ray-induced DNA double-strand breaks (DSB) measured by quantitative analysis of gammaH2AX foci was the same for H1299 cells treated with X-rays with or without Syringetin. CONCLUSIONS: This study supports the hypothesis that Syringetin enhances radiosensitivity more effectively in cancer cells than in normal cells through enhancement of the Caspase-3-mediated apoptosis pathway. Syringetin could be useful in the development of novel efficacious radiosensitizers.
The Methoxyflavonoid Isosakuranetin Suppresses UV-B-Induced Matrix Metalloproteinase-1 Expression and Collagen Degradation Relevant for Skin Photoaging.[Pubmed:27598131]
Int J Mol Sci. 2016 Sep 1;17(9). pii: ijms17091449.
Solar ultraviolet (UV) radiation is a main extrinsic factor for skin aging. Chronic exposure of the skin to UV radiation causes the induction of matrix metalloproteinases (MMPs), such as MMP-1, and consequently results in alterations of the extracellular matrix (ECM) and skin photoaging. Flavonoids are considered as potent anti-photoaging agents due to their UV-absorbing and antioxidant properties and inhibitory activity against UV-mediated MMP induction. To identify anti-photoaging agents, in the present study we examined the preventative effect of methoxyflavonoids, such as sakuranetin, isosakuranetin, homoeriodictyol, genkwanin, chrysoeriol and Syringetin, on UV-B-induced skin photo-damage. Of the examined methoxyflavonoids, pretreatment with isosakuranetin strongly suppressed the UV-B-mediated induction of MMP-1 in human keratinocytes in a concentration-dependent manner. Isosakuranetin inhibited UV-B-induced phosphorylation of mitogen-activated protein kinase (MAPK) signaling components, ERK1/2, JNK1/2 and p38 proteins. This result suggests that the ERK1/2 kinase pathways likely contribute to the inhibitory effects of isosakuranetin on UV-induced MMP-1 production in human keratinocytes. Isosakuranetin also prevented UV-B-induced degradation of type-1 collagen in human dermal fibroblast cells. Taken together, our findings suggest that isosakuranetin has the potential for development as a protective agent for skin photoaging through the inhibition of UV-induced MMP-1 production and collagen degradation.
Changes in grape polyphenols (V. vinifera L.) as a consequence of post-harvest withering by high-resolution mass spectrometry: Raboso Piave versus Corvina.[Pubmed:27491020]
J Mass Spectrom. 2016 Sep;51(9):750-60.
Grape dehydration is an oenological process used for the production of high-quality reinforced and sweet wines. Corvina and Raboso Piave are two red grape varieties used for production of high-quality Italian wines, such as Recioto, Amarone di Valpolicella and Raboso Passito. Changes of polyphenolic composition of the grapes as a consequence of the withering were studied by ultra-high performance liquid chromatography-quadrupole time of flight mass spectrometry (UHPLC/QTOF); for identification of compounds a homemade HR-MS database of grape and wine metabolites, was used. Concomitant with trans-resveratrol and viniferins, relevant increases of other stilbenes (piceatannol, resveratrol trimers and tetramers) and antioxidant compounds (quercetin, Syringetin and tamarixetin) were observed. These compounds are part of the induced metabolism occurring during the withering process and in general improve the nutraceutical properties of grapes and wines. On the other hand, longer processes showed to decrease flavan-3-ols and glycoside flavonols. Constant increase of E/Z epsilon-viniferin ratio was observed in all samples, and this parameter can be used to monitor the process. Copyright (c) 2016 John Wiley & Sons, Ltd.
Cell-based and in silico evidence against quercetin and structurally-related flavonols as activators of vitamin D receptor.[Pubmed:27041117]
J Steroid Biochem Mol Biol. 2016 Oct;163:59-67.
It has been reported that quercetin is an activator of rat vitamin D receptor (rVDR). However, the conclusion was based on experiments performed without all the appropriate control groups, raising the possibility of a false-positive finding. Furthermore, distinct differences exist in the chemical structures of quercetin and 1alpha,25-dihydroxyvitamin D3, which is a prototypic agonist of VDR. Therefore, we investigated systematically whether quercetin and other flavonols are agonists of rVDR, mouse VDR (mVDR), or human VDR (hVDR). Quercetin, 3-hydroxyflavone, galangin, datiscetin, kaempferol, morin, isorhamnetin, tamarixetin, myricetin, and Syringetin did not activate rVDR, mVDR, or hVDR in HEK-293 and HepG2 cells transfected with the corresponding receptor expression plasmid and either the secreted phosphoprotein 1 (Spp1) or cytochrome P450 24A1 (CYP24A1) reporter plasmid, when compared to the respective empty vector control group transfected with one or the other reporter plasmid and treated with one of the flavonols. Control analysis indicated that lithocholic acid and 1alpha,25-dihydroxyvitamin D3, but not rifampicin, activated rVDR, mVDR, and hVDR. As shown in transfected HEK293 and HepG2 cells, the flavonols did not influence hVDR ligand binding domain transactivation, steroid receptor coactivator-1 recruitment, or hVDR target gene expression (transient receptor potential cation channel 6 and CYP24A1) in hVDR-expressing Caco-2 or LS180 cells. The cumulative data from the cell-based experiments were corroborated by results obtained from molecular docking analysis. In conclusion, quercetin, 3-hydroxyflavone, galangin, datiscetin, kaempferol, morin, isorhamnetin, tamarixetin, myricetin, and Syringetin are not agonists of rVDR, mVDR, or hVDR, as judged by cell-based and in silico evidence.
Screening of minor bioactive compounds from herbal medicines by in silico docking and the trace peak exposure methods.[Pubmed:26852619]
J Chromatogr A. 2016 Mar 4;1436:91-9.
Screening of high potent enzyme inhibitors from herbal medicines is always lacking of efficiency due to the complexity of chemicals. The constituents responsible for the holistic effect may be trace-level chemicals, but these chemicals were covered by highly abundant compositions. To challenge this bottleneck, a strategy for screening minor bioactive compounds was proposed. It generally included four steps, (1) preliminarily find the enzyme binders by ultrafiltration; (2) optimise and predict the potential inhibitors by docking analysis; (3) selectively identify and prepare trace compounds by segment and exposure approach; (4) validate the activity and summarize the structure-activity relationship. As a case study, alpha-glucosidase (AGH) and Ginkgo biloba extract were used as the experimental materials. By comprehensive screening, 11 trace flavones were screened out and identified as strong AGH inhibitors. Among them, AGH inhibitory activities of Syringetin and sciadopitysin were reported for the first time. Their IC50 values were 36.80 and 8.29muM, respectively, which were lower than that of a positive control acarbose. In addition, the AGH inhibitory activities of the flavonoids could be ranked, based on a decreased order, as biflavone, flavone, flavone glycoside, flavone biglycoside. The strategy is expected to be practical and useful for screening bioactive molecules from herbal medicines, especially for the minor compounds, which will definitely accelerate the discovery of new drug candidates.
Characterization of Non-Anthocyanic Flavonoids in Some Hybrid Red Grape Extracts Potentially Interesting for Industrial Uses.[Pubmed:26445038]
Molecules. 2015 Oct 2;20(10):18095-106.
Previous studies showed that hybrid grapes often have qualitatively and quantitatively higher polyphenolic contents than the common V. vinifera grape varieties. In general, these compounds are studied for grape chemotaxonomy and for nutraceutical purposes due to their relevant antioxidant activity. Non-anthocyanic flavonoid composition of five red hybrid grape varieties produced by crossing of V. vinifera, V. aestivalis, V. cinerea, V. berlandieri, V. labrusca, V. lincecumii, and V. rupestris were studied by liquid chromatography/high-resolution mass spectrometry. Thirty-one compounds were identified, including methylnaringenin, a tetrahydroxy-dimethoxyflavanone-hexoside, two flavonols (quercetin and a pentahydroxyflavone isomer), 20 glycoside flavonols (four quercetin, two myricetin, two kaempferol, three isorhamnetin, one laricitrin, two Syringetin, one kaempferide and two dihydroflavonol derivatives; myricetin-glucoside-glucuronide; myricetin-diglucoside; Syringetin-dihexoside), three flavan-3-ols (-)-epicatechin, (+)-catechin, (-)-epicatechin gallate) and four proantocyanidins (procyanidin B1, procyanidin B2, procyanidin B3 or B4/B5, procyanidin T2 or T3/T4/C1). Seibel 19881, Seyve Villard 12-347 and Seyve Villard 29-399 were particularly rich in polyphenols. These findings emphasize that these grapes are especially interesting for the production of antioxidant extracts for nutraceutical and pharmaceutical uses.
Methylated derivatives of myricetin enhance life span in Caenorhabditis elegans dependent on the transcription factor DAF-16.[Pubmed:26281763]
Food Funct. 2015 Oct;6(10):3383-92.
Only certain flavonoids have been shown to enhance life span. This was pointed out for e.g. myricetin in the nematode Caenorhabditis elegans. However, the structural requirements responsible for this effect are not known. We used methylated derivatives of myricetin (laricitrin, Syringetin, myricetintrimethylether) to investigate if free OH moieties in the B-ring are necessary for the life span extending effect. In analogy to myricetin, all derivatives increased the life span, decreased oxidative stress (DCF) and decreased the accumulation of lipofuscin. In contrast to myricetin, the methylated compounds strongly enhanced the resistance against thermal stress. Furthermore, treatment with the derivatives induced a much stronger nuclear localization of the DAF-16 transcription factor (FoxO homologue). Additionally, no antioxidant effects and only minor effects on life span prolongation and stress resistance were detectable for the methylated compounds in a DAF-16 deficient nematode strain. Comparable to the dietary flavonoid myricetin, the methylated myricetin derivatives laricitrin, Syringetin and myricetintrimethylether strongly enhance the life span of C. elegans. Therefore, OH groups of ring B are not necessary for this effect. Only the methylated compounds increase the stress resistance of the nematode which was dependent on DAF-16. These findings suggest that methylation of myricetin increases the biofunctionality.
3-Hydroxyflavone and structural analogues differentially activate pregnane X receptor: Implication for inflammatory bowel disease.[Pubmed:26238175]
Pharmacol Res. 2015 Oct;100:64-72.
Pregnane X receptor (PXR; NR1I2) is a member of the superfamily of nuclear receptors that regulates the expression of genes involved in various biological processes, including drug transport and biotransformation. In the present study, we investigated the effect of 3-hydroxyflavone and its structurally-related analogues on PXR activity. 3-Hydroxyflavone, galangin, kaempferol, querceetin, isorhamnetin, and tamarixetin, but not but not datiscetin, morin, myricetin, or Syringetin, activated mouse PXR, as assessed in a cell-based reporter gene assay. By comparison, 3-hydroxyflavone activated rat PXR, whereas 3-hydroxyflavone, galangin, quercetin, isorhamnetin, and tamarixetin activated human PXR (hPXR). A time-resolved fluorescence resonance energy transfer competitive ligand-binding assay showed binding to the ligand-binding domain of hPXR by 3-hydroxyflavone, galangin, quercetin, isorhamnetin, and tamarixetin. 3-Hydroxyflavone and galangin, but not quercetin, isorhamnetin, or tamarixetin, recruited steroid receptor coactivator (SRC)-1, SRC-2, and SRC-3 to hPXR. In LS180 human colon adenocarcinoma cells, 3-hydroxyflavone, quercetin, and tamarixetin increased CYP3A4, CYP3A5, and ABCB1 mRNA expression, whereas galangin and isorhamnetin increased CYP3A4 and ABCB1 but not CYP3A5 mRNA expression. Datiscetin, kaempferol, morin, myricetin, and Syringetin did not attenuate the extent of hPXR activation by rifampicin, suggesting they are not hPXR antagonists. Overall, flavonols activate PXR in an analogue-specific and species-dependent manner. Substitution at the C2' or C5' position of 3-hydroxyflavone with a hydroxyl or methoxy group rendered it incapable of activating hPXR. Understanding the structure-activity relationship of flavonols in hPXR activation may facilitate nutraceutical development efforts in the treatment of PXR-associated intestinal diseases, such as inflammatory bowel disease.


