CannabiscitrinCAS# 520-14-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 520-14-9 | SDF | Download SDF |
| PubChem ID | 5486615 | Appearance | Powder |
| Formula | C21H20O13 | M.Wt | 480.4 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
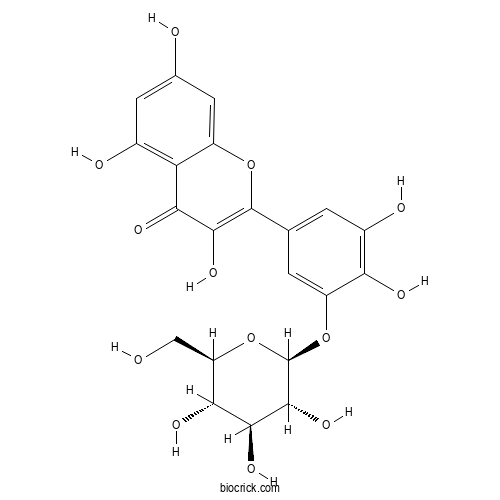
| Chemical Name | 2-[3,4-dihydroxy-5-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyphenyl]-3,5,7-trihydroxychromen-4-one | ||
| SMILES | C1=C(C=C(C(=C1O)O)OC2C(C(C(C(O2)CO)O)O)O)C3=C(C(=O)C4=C(C=C(C=C4O3)O)O)O | ||
| Standard InChIKey | ZJYAVUPWMNHHEU-GFOOFYSOSA-N | ||
| Standard InChI | InChI=1S/C21H20O13/c22-5-12-15(27)17(29)19(31)21(34-12)33-11-2-6(1-9(25)14(11)26)20-18(30)16(28)13-8(24)3-7(23)4-10(13)32-20/h1-4,12,15,17,19,21-27,29-31H,5H2/t12-,15-,17+,19-,21-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Cannabiscitrin Dilution Calculator

Cannabiscitrin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0816 mL | 10.408 mL | 20.816 mL | 41.632 mL | 52.04 mL |
| 5 mM | 0.4163 mL | 2.0816 mL | 4.1632 mL | 8.3264 mL | 10.408 mL |
| 10 mM | 0.2082 mL | 1.0408 mL | 2.0816 mL | 4.1632 mL | 5.204 mL |
| 50 mM | 0.0416 mL | 0.2082 mL | 0.4163 mL | 0.8326 mL | 1.0408 mL |
| 100 mM | 0.0208 mL | 0.1041 mL | 0.2082 mL | 0.4163 mL | 0.5204 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Datiscin
Catalog No.:BCN9869
CAS No.:16310-92-2
- 15-Methoxy-16-oxo-15,16H-strictic acid
Catalog No.:BCN9868
CAS No.:1356388-38-9
- 1,2-Dimethoxybenzene
Catalog No.:BCN9867
CAS No.:91-16-7
- Tropic acid
Catalog No.:BCN9866
CAS No.:529-64-6
- 4-(4-Methoxyphenyl)-2-butanone
Catalog No.:BCN9865
CAS No.:104-20-1
- ICG-001
Catalog No.:BCN9864
CAS No.:780757-88-2
- 3,29-O-Dibenzoyloxykarounidiol
Catalog No.:BCN9863
CAS No.:389122-01-4
- Somnifericin
Catalog No.:BCN9862
CAS No.:173693-57-7
- Solanidine
Catalog No.:BCN9861
CAS No.:80-78-4
- Sphenanlignan
Catalog No.:BCN9860
CAS No.:866347-36-6
- Vanillic acid glucoside
Catalog No.:BCN9859
CAS No.:32142-31-7
- alpha-Peltatin
Catalog No.:BCN9858
CAS No.:568-53-6
- Lappaconine
Catalog No.:BCN9871
CAS No.:23943-93-3
- Condurango glycoside A
Catalog No.:BCN9872
CAS No.:11051-90-4
- 2-(4-Hydroxybenzal)acetophenone
Catalog No.:BCN9873
CAS No.:20426-12-4
- Norharman
Catalog No.:BCN9874
CAS No.:244-63-3
- Syringetin
Catalog No.:BCN9875
CAS No.:4423-37-4
- 4-Hydroxyisophthalic acid
Catalog No.:BCN9876
CAS No.:636-46-4
- Lyciumamide B
Catalog No.:BCN9877
CAS No.:1647111-41-8
- Tricaprin
Catalog No.:BCN9878
CAS No.:621-71-6
- Dihydroquinine
Catalog No.:BCN9879
CAS No.:522-66-7
- Cymarin
Catalog No.:BCN9880
CAS No.:508-77-0
- 5,7-Dihydroxy 3,3',4',5',6,8-hexamethoxyflavone
Catalog No.:BCN9881
CAS No.:96887-18-2
- gamma-Decalactone
Catalog No.:BCN9882
CAS No.:706-14-9
Bioactive Compounds from Abelmoschus manihot L. Alleviate the Progression of Multiple Myeloma in Mouse Model and Improve Bone Marrow Microenvironment.[Pubmed:32099399]
Onco Targets Ther. 2020 Jan 31;13:959-973.
Purpose: Abelmoschus manihot (L.) Medik. (Malvaceae) derived Huangkui capsules (HKC) represent a traditional Chinese medicine that has been widely applied to the clinical therapy of kidney and inflammatory diseases. The present study aimed to determine the potential therapeutic effects and underlying mechanisms of the ingredients on Multiple Myeloma (MM), an incurable disease that exhibits malignant plasma cell clonal expansion in the bone marrow. Methods: A 5TMM3VT syngeneic MM-prone model was established and treated with HKC. Murine pre-osteoblast MC3T3-E1 and pre-osteoclast Raw264.7 cells were treated with nine flavonoid compounds extracted from the flowers of Abelmoschus manihot. MC3T3-E1 and Raw264.7 cells were then examined by alizarin red staining and tartrate-resistant acid phosphatase activity staining, respectively. The proliferation of two human MM cells (ARP1, H929) was examined by performing an MTT assay following treatment with flavonoid compounds. Additionally, the cell cycle was analyzed via staining and flow cytometry. The differential expressions of certain proteins were detected via Western blotting, transcriptomic RNA-sequencing as well as RT-qPCR. Results: The results revealed that MM-prone animals appeared to be protected following HKC treatment, as evidenced by a prolonged survival rate. Furthermore, four of the nine flavonoid compounds [Hyperin/Hyperoside, HK-2; Cannabiscitrin, HK-3; 3-O-kaempferol-3-O-acetyl-6-O-(p-coumaroyl)-beta-D-glucopyranoside, HK-11; 8-(2''-pyrrolidione-5''-yl)-quercetin, HK-B10] induced the differentiation of murine pre-osteoblast MC3T3-E1 cells. In addition, two compounds [Isomyricitrin, HK-8; quercetin-8-(2''-pyrrolidione-5"-yl)-3'-O-beta-D-glucopyranosid, HK-E3] suppressed osteoclastogenesis in murine Raw264.7 cells. HK-11 directly inhibited MM cells (ARP1 and H929) proliferation and induced G0/G1 cell cycle arrest, which may have involved the suppressing beta-catenin protein, increasing expressions of IL-6 and TNF-alpha, as well as activating mature TGF-beta1 and some other metabolic pathways. Conclusion: These results of the present study indicated that the bio-active ingredients of HKC exerted protective effects on MM mouse survival through promoting osteoblastogenesis and suppressing osteoclastogenesis, thus improving the bone marrow microenvironment to inhibit MM cell proliferation.
[Studies on chemical constituents in flower of Abelmoschus manihot].[Pubmed:17165583]
Zhongguo Zhong Yao Za Zhi. 2006 Oct;31(19):1597-600.
OBJECTIVE: To study the chemical constituents of Abelmoschus manihot. METHOD: Chromatographic methods were used to isolate compounds from A. manihot, and spectroscopic methods were used to identify the structures. RESULT: Thirteen compounds, myricetin (1), Cannabiscitrin (2), myricetin-3-O-beta-D-glucopyranoside (3), glycerolmonopalmitate (4), 2, 4-dihydroxy benzoic acid (5), guanosine (6), adenosine (7), maleic acid (8), heptatriacontanoic acid (9), 1-triacontanol (10) , tetracosane (11), beta-sitosterol (12), beta-sitosterol-3-O-beta-D-glucoside (13) were obtained. CONCLUSION: 2-11 were obtained from the genus for the first time.


