SolanidineCAS# 80-78-4 |
Quality Control & MSDS
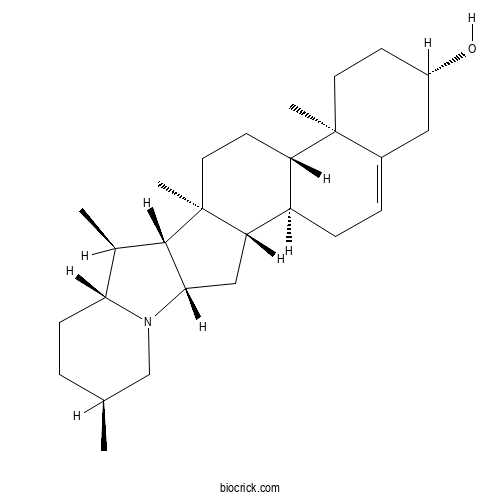
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 80-78-4 | SDF | Download SDF |
| PubChem ID | 65727 | Appearance | White powder |
| Formula | C27H43NO | M.Wt | 397.6 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Synonyms | Solatubine | ||
| Solubility | soluble in methanol, ethanol and DMSO; insoluble in water | ||
| Chemical Name | (1S,2S,7S,10R,11S,14S,15R,16S,17R,20S,23S)-10,14,16,20-tetramethyl-22-azahexacyclo[12.10.0.02,11.05,10.015,23.017,22]tetracos-4-en-7-ol | ||
| SMILES | CC1CCC2C(C3C(N2C1)CC4C3(CCC5C4CC=C6C5(CCC(C6)O)C)C)C | ||
| Standard InChIKey | JVKYZPBMZPJNAJ-OQFNDJACSA-N | ||
| Standard InChI | InChI=1S/C27H43NO/c1-16-5-8-23-17(2)25-24(28(23)15-16)14-22-20-7-6-18-13-19(29)9-11-26(18,3)21(20)10-12-27(22,25)4/h6,16-17,19-25,29H,5,7-15H2,1-4H3/t16-,17+,19-,20+,21-,22-,23+,24-,25-,26-,27-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Reference standards. | |||||

Solanidine Dilution Calculator

Solanidine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5151 mL | 12.5755 mL | 25.1509 mL | 50.3018 mL | 62.8773 mL |
| 5 mM | 0.503 mL | 2.5151 mL | 5.0302 mL | 10.0604 mL | 12.5755 mL |
| 10 mM | 0.2515 mL | 1.2575 mL | 2.5151 mL | 5.0302 mL | 6.2877 mL |
| 50 mM | 0.0503 mL | 0.2515 mL | 0.503 mL | 1.006 mL | 1.2575 mL |
| 100 mM | 0.0252 mL | 0.1258 mL | 0.2515 mL | 0.503 mL | 0.6288 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Sphenanlignan
Catalog No.:BCN9860
CAS No.:866347-36-6
- Vanillic acid glucoside
Catalog No.:BCN9859
CAS No.:32142-31-7
- alpha-Peltatin
Catalog No.:BCN9858
CAS No.:568-53-6
- 5,6-Benzoflavone
Catalog No.:BCN9857
CAS No.:6051-87-2
- Quasipanaxatriol
Catalog No.:BCN9856
CAS No.:171903-78-9
- n-Tridecane
Catalog No.:BCN9855
CAS No.:629-50-5
- 3-Hydroxybenzoic acid
Catalog No.:BCN9854
CAS No.:99-06-9
- 2-Benzal-4-hydroxyacetophenone
Catalog No.:BCN9853
CAS No.:2657-25-2
- Colchiceine
Catalog No.:BCN9852
CAS No.:477-27-0
- Helveticoside
Catalog No.:BCN9851
CAS No.:630-64-8
- Flavonol
Catalog No.:BCN9850
CAS No.:577-85-5
- alpha-Ionone
Catalog No.:BCN9849
CAS No.:127-41-3
- Somnifericin
Catalog No.:BCN9862
CAS No.:173693-57-7
- 3,29-O-Dibenzoyloxykarounidiol
Catalog No.:BCN9863
CAS No.:389122-01-4
- ICG-001
Catalog No.:BCN9864
CAS No.:780757-88-2
- 4-(4-Methoxyphenyl)-2-butanone
Catalog No.:BCN9865
CAS No.:104-20-1
- Tropic acid
Catalog No.:BCN9866
CAS No.:529-64-6
- 1,2-Dimethoxybenzene
Catalog No.:BCN9867
CAS No.:91-16-7
- 15-Methoxy-16-oxo-15,16H-strictic acid
Catalog No.:BCN9868
CAS No.:1356388-38-9
- Datiscin
Catalog No.:BCN9869
CAS No.:16310-92-2
- Cannabiscitrin
Catalog No.:BCN9870
CAS No.:520-14-9
- Lappaconine
Catalog No.:BCN9871
CAS No.:23943-93-3
- Condurango glycoside A
Catalog No.:BCN9872
CAS No.:11051-90-4
- 2-(4-Hydroxybenzal)acetophenone
Catalog No.:BCN9873
CAS No.:20426-12-4
A highly sensitive quantification method for 12 plant toxins in human serum using liquid chromatography tandem mass spectrometry with a quick solid-phase extraction technique.[Pubmed:33099112]
J Pharm Biomed Anal. 2020 Oct 9;192:113676.
We developed a highly sensitive quantification method using liquid chromatography tandem mass spectrometry (LC/MS/MS) for 12 plant toxins in human serum. In this paper, we selected lycorine, galanthamine, protoveratrine A, protoveratrine B, veratramine, veratridine, jervine, cyclopamine, cevadine, alpha-solanine, alpha-chaconine, and Solanidine as targeted analytes. The ADME column was utilized for LC separation and a Monolithic SPE column (MonoSpin(R) C18) for analyte extraction. The total time for SPE clean-up and LC/MS/MS analysis was completed within 30 min. The method validation results were as follows: the linearity (r(2)) of each calibration curve was over 0.99; the inter- and intra-day accuracies were 92.7 %-116 % and 91.6 %-106 %, respectively; and the inter- and intra-day precisions were below 14 % and 11 %, respectively. Also, the lower limits of detection and quantification were 0.0071-0.15 and 0.022-0.46 ng/mL, respectively, indicating the method's high sensitivity. Finally, to confirm its feasibility, our method was applied to two model samples: (1) commercially available human serum and (2) pseudo poisoning serum via dilution of mouse serum with human serum. We were able to quantify alpha-chaconine at 0.84 +/- 0.02 ng/mL in the serum (Case 1) and protoveratrine A at 0.15 +/- 0.032 ng/mL in the pseudo poisoning serum (Case 2), demonstrating our method's practicality. This is the first time that the 12 plant toxins in human serum were simultaneously quantitated. Our method can investigate accidental poisonings involving toxic plants, enabling prompt decisions on patient treatment.
Mediation of Potato-Potato Cyst Nematode, G. rostochiensis Interaction by Specific Root Exudate Compounds.[Pubmed:32587595]
Front Plant Sci. 2020 Jun 10;11:649.
Potato (Solanum tuberosum) is a widely consumed staple food crop worldwide whose production is threatened by potato cyst nematodes (PCN). To infect a host, PCN eggs first need to be stimulated to hatch by chemical components in the host root exudates, yet it remains unknown how most root exudate components influence PCN behavior. Here, we evaluated the influence of eight compounds identified by LC-QqQ-MS in the root exudate of potato on the hatching response of the PCN, Globodera rostochiensis at varying doses. The eight compounds included the amino acids tyrosine, tryptophan and phenylalanine; phytohormones zeatin and methyl dihydrojasmonate; steroidal glycoalkaloids alpha-solanine and alpha-chaconine and the steroidal alkaloid Solanidine. We additionally tested two other Solanaceae steroidal alkaloids, solasodine and tomatidine, previously identified in the root exudates of tomato, an alternative host for PCN. In dose-response assays with the individual compounds, the known PCN hatching factors alpha-chaconine and alpha-solanine stimulated the highest number of eggs to hatch, approximately 47 and approximately 42%, respectively, whereas the steroidal alkaloids (aglycones), Solanidine and solasodine and potato root exudate (PRE) were intermediate, 28% each and 21%, respectively, with tomatidine eliciting the lowest hatching response 13%. However, approximately 60% of the hatched juveniles failed to emerge from the cyst, which was compound- and concentration-dependent. The amino acids, phytohormones and the negative control (1% DMSO in water), however, were generally non-stimulatory. The use of steroidal glycoalkaloids and their aglycones in the suicidal hatching of PCN offers promise as an environmentally sustainable approach to manage this pest.
Correction: Asymmetric synthesis of (-)-solanidine and (-)-tomatidenol.[Pubmed:32441732]
Org Biomol Chem. 2020 Jun 7;18(21):4114.
Correction for 'Asymmetric synthesis of (-)-Solanidine and (-)-tomatidenol' by Yun Wang et al., Org. Biomol. Chem., 2020, 18, 3169-3176, DOI: .
Liquid Chromatography Mass Spectrometry Quantification of alpha-solanine, alpha-chaconine, and Solanidine in Potato Protein Isolates.[Pubmed:32252270]
Foods. 2020 Apr 2;9(4). pii: foods9040416.
For potato proteins to be used as a food ingredient, the level of natural potato defense substances, the glycoalkaloids (GAs), should be limited. In this work, a method is developed for quantification of the two major potato GAs, alpha-solanine and alpha-chaconine, as well as for their aglycon form, Solanidine, using liquid chromatography-mass spectrometry single quadrupole in single ion monitoring mode. Standard solutions of GA and a food-grade potato protein powder was used to validate the method. A linear correlation between GA concentration and the ion intensity of >0.995 was obtained for all standard solutions. Recovery of GA in spiked samples was within the range 82%-106%. The method for GA quantification was applied to a variety of potato protein isolates. The results showed that total GA increased during the storage of the potatoes. Washing the potato protein isolates using water at a sufficient level was shown to be able to reduce the amount of GA below the threshold of 150 microg g(-1), as needed for human consumption.
Discovery of a Bacterial Gene Cluster for Deglycosylation of Toxic Potato Steroidal Glycoalkaloids alpha-Chaconine and alpha-Solanine.[Pubmed:31935098]
J Agric Food Chem. 2020 Feb 5;68(5):1390-1396.
Potato juice is a byproduct of starch processing currently used as feed. However, potato proteins are an untapped source of high-protein food for human nutrition if harmful constituents notably glycoalkaloids (GAs) are detoxified. The two principle GAs found in potato are alpha-chaconine and alpha-solanine, both consisting of a Solanidine aglycone with a carbohydrate side chain. The first step in the detoxification of these compounds is the removal of the trisaccharide. Whole-genome sequencing of a bacterial isolate, Arthrobacter sp. S41, capable of completely degrading alpha-chaconine and alpha-solanine, revealed the presence of a gene cluster possibly involved in the deglycosylation of GAs. Functional characterization confirmed the enzymatic activity of the gene cluster involved in the complete deglycosylation of both alpha-chaconine and alpha-solanine. The novel enzymes described here may find value in the bioconversion of feed proteins to food proteins suitable for human nutrition.
Growth and alkaloid production along with expression profiles of biosynthetic pathway genes in two contrasting morphotypes of prickly and prickleless Solanum viarum Dunal.[Pubmed:31814043]
Protoplasma. 2020 Mar;257(2):561-572.
Growth and production kinetics of three important glycoalkaloids viz. alpha-solanine, Solanidine, and solasodine in two contrasting prickly and prickleless plants of Solanum viarum Dunal were evaluated under in vitro conditions. The prickleless plants showed improved accumulation of total glycoalkaloid content [7.11 and 6.85 mg g(-1) dry weight (DW)] and growth (GI = 11.08 and 19.26) after 45 and 50 days of culture cycle, respectively. For higher biomass (91.18 g l(-1)) as well as glycoalkaloid (52.56 mg l(-1)) recovery, the prickleless plants served as highly profitable platform. All the three studied glycoalkaloids were identified and quantified by mass spectrometry and HPLC. All the three studied glycoalkaloids accumulated in age-dependent manner. The presence of two constituents, i.e., solasodine and Solanidine mainly contributed for higher accumulation of total glycoalkaloid content in the prickleless plants. However, the synthesis of alpha-solanine was highly age specific and could be detected after 4 to 5 weeks of culture cycle in both prickle containing as well as prickleless plants of S. viarum. The higher accumulation of glycoalkaloids in prickleless plants was also supported with the expression analysis of six key pathway enzymes viz. mevalonate kinase (MVK), 3-hydroxy-3-methyl-glutaryl coenzyme A reductase (HMGR), farnesyl diphosphate synthase (FPS), UDP-galactose/Solanidine galactosyltransferase (SGT1), UDP-glucose/Solanidine glucosyltransferase (SGT2), and cytochrome P450 monooxygenase (CYP). The results indicated that the plants harvested after 45 and 50 days of culture cycle accumulated maximum bioactive in-demand glycoalkaloids in the prickly and prickleless plants of S. viarum Dunal, respectively.
[Studies on alkaloid constituents of Fritillaria yuminensis].[Pubmed:30989914]
Zhongguo Zhong Yao Za Zhi. 2019 Feb;44(3):495-499.
Twelve alkaloids were isolated from the bulbs of Fritillaria yuminensis by column chromatography over silica gel, ODS, and Sephadex LH-20, as well as RP-HPLC. Their structures were identified mainly by NMR and MS analyses as yubeinine(1), imperialine(2), delavinone(3), tortifoline(4), hupehenizioiside(5), imperialine-beta-D-glucoside(6), kuroyurinidine(7), pengbeisine A(8), walujewine A(9), peimisine-3-O-beta-D-glucopyranoside(10), Solanidine-3-O-alpha-L-rhamnopyranosyl-(1-->2)-beta-D-glucopyranoside(11), and Solanidine-3-O-alpha-L-rhamnopyranosyl-(1-->2)-[beta-D-glucopyranosyl-(1-->4)]-be ta-D-glucopyranoside(12). Compounds 4-12 were obtained from F. yuminensis for the first time.
Biomarkers of tuber intake.[Pubmed:30984301]
Genes Nutr. 2019 Apr 2;14:9.
Tubers are important crops as well as staple foods in human nutrition. Among tubers, the potato in particular has been investigated for its health effects. However, except for its contribution to energy and effects related to resistant starch, the role of potatoes and other tubers in human health is still debated. In order to establish firm evidence for the health effects of dietary tubers and processed tuber products, it is essential to assess total intake accurately. The dietary assessment in most studies relies mainly on self-reporting and may give imprecise quantitative information on dietary intakes. Biomarkers of food intake (BFIs) are useful objective means to assess intake of specific foods or may be used as an additional measure to calibrate the measurement error in dietary reports. Here, intake biomarkers for common tubers, including potatoes and heated potato products, sweet potato, cassava, yam, and Jerusalem artichoke, are reviewed according to the biomarker of food intake reviews (BFIRev) standardized protocols for review and validation. Candidate BFIs for heated potato product include alpha-chaconine, alpha-solanine, and Solanidine; less evidence is available to indicate peonidin 3-caffeoylsophoroside-5-glucoside and cyanidin 3-caffeoylsophoroside-5-glucoside as putative biomarkers having high potential specificity for purple sweet potato intake; linamarin may in addition be considered as a putative BFI for cassava. Other tubers also contain toxic glycosides or common contaminants as characteristic components but their putative use as intake biomarkers is not well documented. Alkyl pyrazines, acrylamide, and acrolein are formed during cooking of heated potato products while these have not yet been investigated for other tubers; these markers may not be specific only to heated potato but measurements of these compounds in blood or urine may be combined with more specific markers of the heated products, e.g., with glycoalkaloids to assess heated potato products consumption. Further studies are needed to assess the specificity, robustness, reliability, and analytical performance for the candidate tuber intake biomarkers identified in this review.
Steroidal glycoalkaloids from Solanum nigrum target cytoskeletal proteins: an in silico analysis.[Pubmed:30627484]
PeerJ. 2019 Jan 3;7:e6012.
Background: Solanum nigrum (black nightshade; S. nigrum), a member of family Solanaceae, has been endowed with a heterogeneous array of secondary metabolites of which the steroidal glycoalkaloids (SGAs) and steroidal saponins (SS) have vast potential to serve as anticancer agents. Since there has been much controversy regarding safety of use of glycoalkaloids as anticancer agents, this area has remained more or less unexplored. Cytoskeletal proteins like actin play an important role in maintaining cell shape, synchronizing cell division, cell motility, etc. and along with their accessory proteins may also serve as important therapeutic targets for potential anticancer candidates. In the present study, glycoalkaloids and saponins from S. nigrum were screened for their interaction and binding affinity to cytoskeletal proteins, using molecular docking. Methods: Bioactivity score and Prediction of Activity Spectra for Substances (PASS) analysis were performed using softwares Molinspiration and Osiris Data Explorer respectively, to assess the feasibility of selected phytoconstituents as potential drug candidates. The results were compared with two standard reference drugs doxorubicin hydrochloride (anticancer) and tetracycline (antibiotic). Multivariate data obtained were analyzed using principal component analysis (PCA). Results: Docking analysis revealed that the binding affinities of the phytoconstituents towards the target cytoskeletal proteins decreased in the order coronin>villin>ezrin>vimentin>gelsolin>thymosin>cofilin. Glycoalkaloid solasonine displayed the greatest binding affinity towards the target proteins followed by alpha-solanine whereas amongst the saponins, nigrumnin-I showed maximum binding affinity. PASS Analysis of the selected phytoconstituents revealed 1 to 3 violations of Lipinski's parameters indicating the need for modification of their structure-activity relationship (SAR) for improvement of their bioactivity and bioavailability. Glycoalkaloids and saponins all had bioactivity scores between -5.0 and 0.0 with respect to various receptor proteins and target enzymes. Solanidine, solasodine and solamargine had positive values of druglikeness which indicated that these compounds have the potential for development into future anticancer drugs. Toxicity potential evaluation revealed that glycoalkaloids and saponins had no toxicity, tumorigenicity or irritant effect(s). SAR analysis revealed that the number, type and location of sugar or the substitution of hydroxyl group on alkaloid backbone had an effect on the activity and that the presence of alpha-L-rhamnopyranose sugar at C-2 was critical for a compound to exhibit anticancer activity. Conclusion: The present study revealed some cytoskeletal target(s) for S. nigrum phytoconstituents by docking analysis that have not been previously reported and thus warrant further investigations both in vitro and in vivo.
Synthesis and insecticidal activities of novel solanidine derivatives.[Pubmed:30136365]
Pest Manag Sci. 2019 Mar;75(3):793-800.
BACKGROUND: Potato (Solanum tuberosum) is the fourth culture in the world and is widely used in the agri-food industries. They generate by-products in which alpha-chaconine and alpha-solanine, the two major Solanidine-based glycoalkaloids of potato, are present. As secondary metabolites, they play an important role in the protection system of potato and are involved in plant protection against insects. To add value to these by-products, we described here new glycoalkaloids that could have phytosanitary properties. RESULTS: Solanidine, as a renewable source, was modified with an azido linker and coupled by copper-catalyzed alkyne azide cycloaddition to alkynyl derivatives of the monosaccharides found in the natural potato glycoalkakoids: D-glucose, D-galactose and L-rhamnose. The efficacy of our compounds was evaluated on the potato aphid Macrosiphum euphorbiae. The synthetic compounds have stronger aphicidal properties against nymphs than unmodified Solanidine. They also showed strong aphicidal activities on adults and a negative impact on fecundity. CONCLUSION: Our synthetic neoglycoalkaloids affected Macrosiphum euphorbiae survival at the nymphal stage as well as at the adult stage. Furthermore, they induced a decrease in fecundity. Our results show that chemical modifications of by-products may afford new sustainable compounds for crop and plant protection. (c) 2018 Society of Chemical Industry.
Potato Peels and Their Bioactive Glycoalkaloids and Phenolic Compounds Inhibit the Growth of Pathogenic Trichomonads.[Pubmed:30039703]
J Agric Food Chem. 2018 Aug 1;66(30):7942-7947.
Potato peel, a waste product of the potato processing industry, is high in bioactive compounds. We investigated the in vitro antitrichomonad activity of potato peel powders prepared from commercial Russet, red, purple, and fingerling varieties as well as several known potato components, alkaloids and phenolic compounds, against three pathogenic strains of trichomonads. Trichomonas vaginalis is a sexually transmitted protozoan parasite that causes the human disease trichomoniasis. Two distinct strains of the related Tritrichomonas fetus infect cattle and cats. The glycoalkaloids alpha-chaconine and alpha-solanine were highly active against all parasite lines, while their common aglycone Solanidine was only mildly inhibitory. alpha-Solanine was several times more active than alpha-chaconine. The phenolic compounds caffeic and chlorogenic acids and quercetin were mildly active against the parasites. Most of the potato peel samples were at least somewhat active against all three trichomonad species, but their activities were wide-ranging and did not correspond to their glycoalkaloid and phenolic content determined by HPLC. The two Russet samples were the most active against all three parasites. The purple potato peel sample was highly active against bovine and mostly inactive against feline trichomonads. None of the test substances were inhibitory toward several normal microflora species, suggesting the potential use of the peels for targeted therapeutic treatments against trichomonads.
The tumor antagonistic steroidal alkaloid Solanidine prompts the intrinsic suicidal signal mediated DFF-40 nuclear import and nucleosomal disruption.[Pubmed:29524520]
Life Sci. 2018 Apr 15;199:139-150.
Aim Deformity in the cellular homeostatic event associated with cell survival and apoptosis are committing factors for carcinogenesis. Interventions of these events by pharmacologically active agent gain predominance in cancer treatment. In current investigation Solanidine, a steroidal alkaloid was evaluated on tumorigenesis by targeting death signal using multiple tumor cells and model systems. MAIN METHODS: Anti-proliferative effect was evaluated using cytotoxic studies. Prolonged cytotoxic effect of Solanidine was examined by colony formation assay. Exhibition of apoptotic hallmark induced by Solanidine was examined using FACS analysis, Annexin-V staining, Acridine orange staining, TUNEL assay. Altered gene expression was evaluated using Immunoblot, Immunofluorescence and Immunohistochemistry technique. In-vitro results were revalidated in EAC solid tumor and CAM xenograft model. KEY FINDINGS: Solanidine exerts its potential effect in a target specific manner. The cytotoxic/anticlonogenic activity was due to induction of typical cellular apoptotic hallmarks and cell cycle blockage at S-G2/M phase. The molecular events underlying this effect is through activation of intrinsic pathway via Bax, Bad and Cytochrome c activation by neutralizing Bcl-2 expression, along with downregulated PI3K/Akt survival signal. As a consequence, downstream pro apoptogenic gene, active Caspase-3 was over expressed by Solanidine to cleave its substrate PARP and promotes nuclear import of DFF-40. Anti-carcinogenic aptitude was further confirmed by murine solid tumors and in-vivo CAM xenograft studies. SIGNIFICANCE: Solanidine emerged as active molecule against tomorigenesis by activating nuclear import of DFF-40 mediated nucleosomal disruption and cell demise. It can be developed as a potential apoptogenic small molecule for cancer therapy.
Aglycone solanidine and solasodine derivatives: A natural approach towards cancer.[Pubmed:28779706]
Biomed Pharmacother. 2017 Oct;94:446-457.
Over the past few years, it was suggested that a rational approach to treat cancer in clinical settings requires a multipronged approach that augments improvement in systemic efficiency along with modification in cellular phenotype leads to more efficient cell death response. Recently, the combinatory delivery of traditional chemotherapeutic drugs with natural compounds proved to be astonishing to deal with a variety of cancers, especially that are resistant to chemotherapeutic drugs. The natural compounds not only synergize the effects of chemotherapeutics but also minimize drug associated systemic toxicity. In this review, our primary focus was on antitumor effects of natural compounds. Previously, the drugs from natural sources are highly precise and safer than drugs of synthetic origins. Many natural compounds exhibit anti-cancer potentials by inducing apoptosis in different tumor models, in-vitro and in-vivo. Furthermore, natural compounds are also found equally useful in chemotherapeutic drug resistant tumors. Moreover, these Phyto-compounds also possess numerous other pharmacological properties such as antifungal, antimicrobial, antiprotozoal, and hepatoprotection. Aglycone solasodine and Solanidine derivatives are the utmost important steroidal glycoalkaloids that are present in various Solanum species, are discussed here. These natural compounds are highly cytotoxic against different tumor cell lines. As the molecular weight is concerned; these are smaller molecular weight chemotherapeutic agents that induce cell death response by initiating apoptosis through both extrinsic and intrinsic pathways.
Divergent Synthesis of Solanidine and 22-epi-Solanidine.[Pubmed:28621138]
J Org Chem. 2017 Jul 21;82(14):7463-7469.
A divergent synthesis of Solanidine and 22-epi-Solanidine, two 25S natural steroidal alkaloids, from 25R-configured diosgenin acetate, is described. Initially, Solanidine was synthesized through a series of transformations including a cascade ring-switching process of furostan-26-acid, an epimerization of C25 controlled by the conformation of six-membered lactone ring, an intramolecular Schmidt reaction, and an imine reduction/intramolecular aminolysis process. To address the epimerization issue during Schmidt reaction, an improved synthesis was developed, which also led to a synthesis of 22-epi-Solanidine. In this synthesis, selective transformation of azido lactone to azido diol and amino diol was realized through a reduction relay tactic. The azido diol was transformed to Solanidine via an intramolecular Schmidt reaction/N-alkylation/reduction process and to 22-epi-Solanidine via an intramolecular double N-alkylation process.


