alpha-PeltatinCAS# 568-53-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 568-53-6 | SDF | Download SDF |
| PubChem ID | 92129 | Appearance | White to off-white powder |
| Formula | C21H20O8 | M.Wt | 400.4 |
| Type of Compound | Lignans | Storage | Desiccate at -20°C |
| Solubility | Soluble in chloroform and ethan | ||
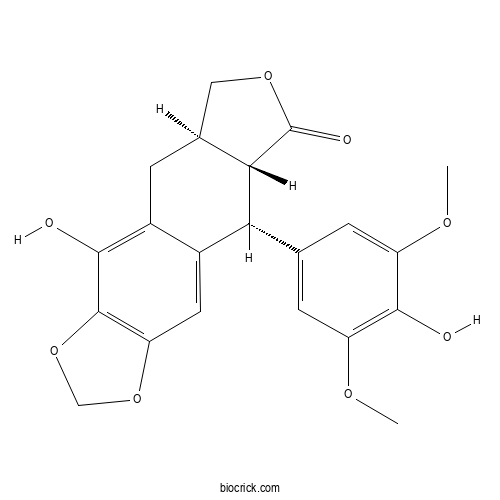
| Chemical Name | (5aR,8aR,9R)-4-hydroxy-9-(4-hydroxy-3,5-dimethoxyphenyl)-5a,6,8a,9-tetrahydro-5H-[2]benzofuro[5,6-f][1,3]benzodioxol-8-one | ||
| SMILES | COC1=CC(=CC(=C1O)OC)C2C3C(CC4=C(C5=C(C=C24)OCO5)O)COC3=O | ||
| Standard InChIKey | JGGWNGRBXJWAOC-HKJPBSJPSA-N | ||
| Standard InChI | InChI=1S/C21H20O8/c1-25-13-4-9(5-14(26-2)19(13)23)16-11-6-15-20(29-8-28-15)18(22)12(11)3-10-7-27-21(24)17(10)16/h4-6,10,16-17,22-23H,3,7-8H2,1-2H3/t10-,16+,17-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | alpha-Peltatin is a lignan podophyllotoxin, it shows phytotoxicity. | |||||

alpha-Peltatin Dilution Calculator

alpha-Peltatin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4975 mL | 12.4875 mL | 24.975 mL | 49.95 mL | 62.4376 mL |
| 5 mM | 0.4995 mL | 2.4975 mL | 4.995 mL | 9.99 mL | 12.4875 mL |
| 10 mM | 0.2498 mL | 1.2488 mL | 2.4975 mL | 4.995 mL | 6.2438 mL |
| 50 mM | 0.05 mL | 0.2498 mL | 0.4995 mL | 0.999 mL | 1.2488 mL |
| 100 mM | 0.025 mL | 0.1249 mL | 0.2498 mL | 0.4995 mL | 0.6244 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 5,6-Benzoflavone
Catalog No.:BCN9857
CAS No.:6051-87-2
- Quasipanaxatriol
Catalog No.:BCN9856
CAS No.:171903-78-9
- n-Tridecane
Catalog No.:BCN9855
CAS No.:629-50-5
- 3-Hydroxybenzoic acid
Catalog No.:BCN9854
CAS No.:99-06-9
- 2-Benzal-4-hydroxyacetophenone
Catalog No.:BCN9853
CAS No.:2657-25-2
- Colchiceine
Catalog No.:BCN9852
CAS No.:477-27-0
- Helveticoside
Catalog No.:BCN9851
CAS No.:630-64-8
- Flavonol
Catalog No.:BCN9850
CAS No.:577-85-5
- alpha-Ionone
Catalog No.:BCN9849
CAS No.:127-41-3
- Gardenin A
Catalog No.:BCN9848
CAS No.:21187-73-5
- Urushiol (15:3)
Catalog No.:BCN9847
CAS No.:83543-37-7
- Vitexin 7-glucoside
Catalog No.:BCN9846
CAS No.:35109-95-6
- Vanillic acid glucoside
Catalog No.:BCN9859
CAS No.:32142-31-7
- Sphenanlignan
Catalog No.:BCN9860
CAS No.:866347-36-6
- Solanidine
Catalog No.:BCN9861
CAS No.:80-78-4
- Somnifericin
Catalog No.:BCN9862
CAS No.:173693-57-7
- 3,29-O-Dibenzoyloxykarounidiol
Catalog No.:BCN9863
CAS No.:389122-01-4
- ICG-001
Catalog No.:BCN9864
CAS No.:780757-88-2
- 4-(4-Methoxyphenyl)-2-butanone
Catalog No.:BCN9865
CAS No.:104-20-1
- Tropic acid
Catalog No.:BCN9866
CAS No.:529-64-6
- 1,2-Dimethoxybenzene
Catalog No.:BCN9867
CAS No.:91-16-7
- 15-Methoxy-16-oxo-15,16H-strictic acid
Catalog No.:BCN9868
CAS No.:1356388-38-9
- Datiscin
Catalog No.:BCN9869
CAS No.:16310-92-2
- Cannabiscitrin
Catalog No.:BCN9870
CAS No.:520-14-9
[A new biflavone from Dysosma versipellis].[Pubmed:29905994]
Yao Xue Xue Bao. 2016 Aug;51(8):1281-4.
This study was conducted to investigate the chemical constituents in the root of Dysosma versipellis(Hance) M. Cheng. The constituents were isolated by silica gel, lichroprep RP-C(18) and pharmadex LH-20 column chromatography and the IR, MS, NMR, 2D-NMR spectroscopic analysis were employed for the structural elucidation. Ten compounds were isolated from the 95% ethanol extract of Dysosma versipellis, their structures were elucidated as dysoverine D (1), dysoverine F (2), dysoverine A (3), podoverine A (4), alpha-Peltatin (5), rutin (6), kaempferol-3-O-beta-D-glucopyranoside (7), quercetin-3-O-beta-D-glucopyranoside (8), kaempferol (9) and quercetin (10). Compound 2 is a new compound, and compounds 1 and 3-6 were isolated from this plant for the first time.
Novel linear and step-gradient counter-current chromatography for bio-guided isolation and purification of cytotoxic podophyllotoxins from Dysosma versipellis (Hance).[Pubmed:23418155]
J Sep Sci. 2013 Mar;36(6):1022-8.
Dysosma versipellis (Hance) is a famous traditional Chinese medicine for the treatment of snakebite, weakness, condyloma accuminata, lymphadenopathy, and tumors for thousands of years. In this work, four podophyllotoxin-like lignans including 4'-demethylpodophyllotoxin (1), alpha-Peltatin (2), podophyllotoxin (3), beta-peltatin (4) as major cytotoxic principles of D. versipellis were successfully isolated and purified by several novel linear and step gradient counter-current chromatography methods using the systems of hexane/ethyl acetate/methanol/water (4:6:3:7 and 4:6:4:6, v/v/v/v). Compared with isocratic elution, linear and step-gradient elution can provide better resolution and save more time for the separation of photophyllotoxin and its congeners. Their cytotoxicities were further evaluated and their structures were validated by high-resolution electrospray TOF MS and nuclear magnetic resonance spectra. All components showed potent anticancer activity against human hepatoma cells HepG2.
Identification of lignans and related compounds in Anthriscus sylvestris by LC-ESI-MS/MS and LC-SPE-NMR.[Pubmed:21889175]
Phytochemistry. 2011 Dec;72(17):2172-9.
The aryltetralin lignan deoxypodophyllotoxin is much more widespread in the plant kingdom than podophyllotoxin. The latter serves as a starting compound for the production of cytostatic drugs like etoposide. A better insight into the occurrence of deoxypodophyllotoxin combined with detailed knowledge of its biosynthestic pathway(s) may help to develop alternative sources for podophyllotoxin. Using HPLC combined with electrospray tandem mass spectrometry and NMR spectroscopy techniques, we found nine lignans and five related structures in roots of Anthriscus sylvestris (L.) Hoffm. (Apiaceae), a common wild plant in temperate regions of the world. Podophyllotoxone, deoxypodophyllotoxin, yatein, anhydropodorhizol, 1-(3'-methoxy-4',5'-methylenedioxyphenyl)1-xi-methoxy-2-propene, and 2-butenoic acid, 2-methyl-4-[[(2Z)-2-methyl-1-oxo-2-buten-1-yl]oxy]-, (2E)-3-(7-methoxy-1,3-benzodioxol-5-yl)-2-propen-1-yl ester, (2Z)- were the major compounds. alpha-Peltatin, podophyllotoxin, beta-peltatin, isopicropodophyllone, beta-peltatin-a-methylether, (Z)-2-angeloyloxymethyl-2-butenoic acid, anthriscinol methylether, and anthriscrusin were present in lower concentrations. alpha-Peltatin, beta-peltatin, isopicropodophyllone, podophyllotoxone, and beta-peltatin-a-methylether have not been previously reported to be present in A. sylvestris. Based on our findings we propose a hypothetical biosynthetic pathway of aryltetralin lignans in A. sylvestris.
Rapid analysis of lignans from leaves of Podophyllum peltatum L. samples using UPLC-UV-MS.[Pubmed:21374649]
Biomed Chromatogr. 2011 Nov;25(11):1230-6.
A new rapid UPLC-UV-MS method has been developed that permits the analysis of four lignans (4'-O-demethylpodophyllotoxin, podophyllotoxin, alpha-Peltatin and beta-peltatin) in P. peltatum L. Podophyllotoxin is a natural lignan that is being used as a precursor for the semi-synthetic anti-cancer drugs etoposide, teniposide and etopophos. The chromatographic separation was achieved using a reversed-phase C18 column with a mobile phase of water and acetonitrile, both containing 0.05% formic acid. Analyses of P. peltatum leaves collected from different colonies within a single site indicated a significant variation in 4'-O-demethylpodophyllotoxin, alpha-Peltatin, podophyllotoxin and beta-peltatin content. Within 3.0 min four main lignans could be separated with detection limits of 0.1, 0.3, 0.3 and 0.2 mug/mL, respectively. 4'-O-demethylpodophyllotoxin and alpha-Peltatin appeared most prominently among the lignans obtained. The podophyllotoxin content was found in the range of 0.004-0.77% from 16 samples collected from 6 colonies within the same site. The content of podophyllotoxin is directly proportional to the content of 4'-O-demethylpodophyllotoxin and inversely proportional to alpha-Peltatin and beta-peltatin content. LC-mass spectrometry coupled with electrospray ionization (ESI) interface method is described for the identification of four lignans in various populations of plant samples. By applying principal component analysis and hierarchical cluster analysis, Podophyllum samples collected from various colonies within a location were distinguished.
Evaluation of Podophyllum peltatum accessions for podophyllotoxin production.[Pubmed:11988859]
Planta Med. 2002 Apr;68(4):341-4.
In an effort to develop a sustainable source of podophyllotoxin for the production of anticancer drugs such as etoposide, teniposide and etopophos, Podophyllum peltatum accessions with podophyllotoxin-rich leaf biomass were identified and transplanted to different growing conditions by vegetative cuttings. Results indicate that the lignan profile in leaves does not change over time or due to environment conditions. Podophyllotoxin and alpha-Peltatin content in the blades seems to be stable with an inverse relationship of concentration between these compounds. A podophyllotoxin-rich leaf accession showed low biosynthetic capability to synthesize alpha- and beta-peltatin and the converse was also true, indicating that selection and cultivation of high-yielding podophyllotoxin leaf biomass may reduce production costs.
Podophyllotoxin lignans enhance IL-1beta but suppress TNF-alpha mRNA expression in LPS-treated monocytes.[Pubmed:11322652]
Immunopharmacol Immunotoxicol. 2001 Feb;23(1):83-95.
There exists a growing body of research which indicates that antimitotics such as taxol and colchicine influence cytokine gene expression. In the present study we examined the effect of podophyllotoxin and six analogs on nuclear factor kappa B (NF-kappa B) activation, and on interleukin-1 beta (IL-1beta) and tumor necrosis factor alpha (TNF-alpha) mRNA expression in human THP-1 monocytes. All compounds were inactive between 0.001microM and 10microM when tested alone. However, podophyllotoxin (0.1 microM) enhanced LPS-induced NF-kappa B activation and IL-1beta mRNA expression between 2 and 3-fold. In contrast, LPS-induced TNF-alpha mRNA expression was decreased between 3 and 6-fold. Comparable results were also observed with the three analogs acetylpodophyllotoxin, 4'-demethylpodophyllotoxin and alpha-Peltatin. The remaining three analogs (podophyllotoxin-4-O-glucoside, beta-peltatin-beta-D-glucopyransoide and 1,2,3,4-dehydrodesoxypodophyllotoxin) were inactive. Clearly certain structural features such as the presence of a glycosidic group or ring aromatization results in loss of biological activity. Interestingly, the analogs that were inactive in our assays have also been previously shown to lack affinity for tubulin binding. These results suggest that during the initial hours of exposure to podophyllotoxin or specific analogs these compounds do not act as independent stimulants of human monocyte activation, but can selectively enhance or suppress LPS-induced cytokine gene expression.
The antiviral action of lignans.[Pubmed:2559420]
Planta Med. 1989 Dec;55(6):531-5.
A total of 18 purified lignans was evaluated for antiviral activity against murine cytomegalovirus (CMV) and Sindbis virus, by means of different treatment regimens. Podophyllotoxin and alpha-Peltatin were the most potent compounds, and they apparently inhibited murine CMV at an essential early step in the replication cycle after the adsorption of virus to the cells. On the other hand, justicidin B and the diphyllin derivatives were much more effective against Sindbis virus, and 12 of the lignans had no demonstrable effect at all, despite their known activities in other bioassays.
Antitumor agents. 100. Inhibition of human DNA topoisomerase II by cytotoxic ether and ester derivatives of podophyllotoxin and alpha-peltatin.[Pubmed:2537424]
J Med Chem. 1989 Mar;32(3):604-8.
A principal mechanism of action of the clinical antitumor drugs etoposide (1) and teniposide (2) is the inhibition of catalytic activity of type II DNA topoisomerase and concurrent enzyme-mediated production of lethal DNA strand breaks. Substitution of the glycosidic moiety of 1 or 2 by ester and ethers, as well as the esterification and etherification of alpha-Peltatin (4) including its glucosidic ethylidene and thenylidene cyclic acetals (25 and 26), has afforded compounds of much less activity than that of 1. The in vitro cytotoxicity (KB) appears to have no correlation with the inhibitory activity of the human DNA topoisomerase II.
Antitumor agents. 78. Inhibition of human DNA topoisomerase II by podophyllotoxin and alpha-peltatin analogues.[Pubmed:3016271]
J Med Chem. 1986 Aug;29(8):1547-50.
It has been reported that the action of etoposide (VP-16) (14) as an antitumor agent is mediated through its interaction with DNA topoisomerase II which results in DNA breakage inside the cell. In order to understand the mechanism of action as well as structure-activity relationships of 14, several novel, synthetic and some naturally occurring analogues related to podophyllotoxin were examined for inhibition of the DNA topoisomerase II activity. Compound 2 exhibited enhanced activity and compound 5 slightly diminished activity relative to 14. A 4 beta-substituted ether at the C ring and O-demethylation at the E ring appear to enhance activity.
Comparison of cytotoxicity and DNA breakage activity of congeners of podophyllotoxin including VP16-213 and VM26: a quantitative structure-activity relationship.[Pubmed:6712942]
Biochemistry. 1984 Mar 13;23(6):1183-8.
Fourteen congeners of podophyllotoxin were evaluated for their abilities to induce DNA breakage and inhibit growth of A549 human lung adenocarcinoma cells. Among the congeners studied were VP16-213, VM26, alpha-Peltatin, beta-peltatin, and picropodophyllotoxin. Alkaline elution methods were used to assess DNA break frequencies following 1-h exposure to different concentrations of the congeners. DNA breakage was dependent upon drug concentration and was detectable when cells were exposed for 1 h to concentrations of VM26 as low as 0.05 microM. DNA breaks formed rapidly in cells after addition of drug but increased little after 30 min of continuous exposure. Repair of drug-induced DNA breaks was equally rapid with repair of 90% of the breaks occurring within 1 h following removal of the drug. Relationships between the structures of the congeners and the resulting DNA breakage activities were obtained, which correlated well with the cytotoxicity. The data suggest that a free hydroxyl group at the 4'-position is essential for DNA breakage activity, epimerization at the 4-position of the podophyllotoxin rings enhances activity, glucosylation of the hydroxyl group at the 4-position diminishes activity, aldehyde condensation with the glucose moiety greatly enhances activity, and the structure of the group associated with the resulting acetal linkage influences DNA breakage activity. These studies present quantitative data supporting and expanding upon the structure-activity relationship first proposed by Loike and Horwitz [Loike, J. D., & Horwitz, S. B. (1976) Biochemistry 15, 5443-5448].
Podophyllotoxin-resistant mutants of Chinese hamster ovary cells: cross-resistance studies with various microtubule inhibitors and podophyllotoxin analogues.[Pubmed:6848174]
Cancer Res. 1983 Feb;43(2):505-12.
The cross-resistances of several mutants of Chinese hamster ovary cells which have been obtained after one and two selection steps in the presence of the microtubule inhibitor podophyllotoxin (PodRI and PodRII mutants, respectively) towards various other inhibitors of microtubule assembly (e.g., colchicine, Colcemid, vinblastine, griseofulvin, maytansine, steganacin, nocodazole, and taxol) have been examined. Based upon their specific patterns of cross-resistance/sensitivity to various microtubule inhibitors, both the PodRI and PodRII classes of mutants appear to be of more than one kind. Studies on the binding of [3H]podophyllotoxin to cytoplasmic extracts indicate that one of the PodRII mutants which has been shown previously to be affected in a Mr 66,000 to 68,000 microtubule-associated protein shows reduced binding of the drug in comparison to the parental PodS and PodRI cells. The different PodRI and PodRII mutants exhibited proportionally increased cross-resistances to various podophyllotoxin analogues (e.g., deoxypodophyllotoxin, epipodophyllotoxin, beta-peltatin, 4'-demethylpodophyllotoxin, alpha-Peltatin, podophyllotoxin-beta-D-glucoside, beta-peltatin-beta-D-glucoside, picropodophyllotoxin, and podophyllic acid) which possess microtubule-inhibitory activity. However, with the exception of one PodRI class of mutant, none of the mutants exhibited any cross-resistance to 4'-demethylepipodophyllotoxin thenylidine-beta-D-glucoside and 4'-demethylepipodophyllotoxin ethylidine-beta-D-glucoside, the 2 podophyllotoxin analogues which lack microtubule-inhibitory activity. The cross-resistance studies with these mutants, which, based upon the biochemical studies and their highly specific patterns of cross-resistance, are presumably affected in microtubules, provide some very novel insights into the mechanisms of action of various microtubule inhibitors. The results presented in this paper also show that the cross-resistance studies with the set of podophyllotoxin-resistant mutants provide a sensitive and highly specific screening procedure for identifying compounds which possess podophyllotoxin-like activity and for investigating the structure-activity relationship among them. The results of structure-activity relationship studies for the various podophyllotoxin analogues examined are discussed.


