ICG-001CAS# 780757-88-2 |
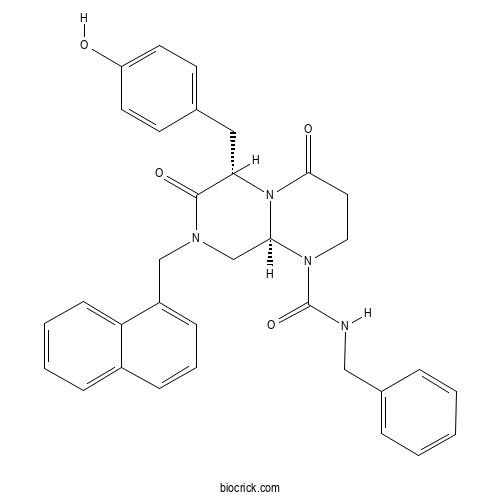
- ICG 001
Catalog No.:BCC3632
CAS No.:847591-62-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 780757-88-2 | SDF | Download SDF |
| PubChem ID | 11238147 | Appearance | Powder |
| Formula | C33H32N4O4 | M.Wt | 548.63 |
| Type of Compound | Inhibitors | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (6S,9aS)-N-benzyl-6-[(4-hydroxyphenyl)methyl]-8-(naphthalen-1-ylmethyl)-4,7-dioxo-3,6,9,9a-tetrahydro-2H-pyrazino[1,2-a]pyrimidine-1-carboxamide | ||
| SMILES | C1CN(C2CN(C(=O)C(N2C1=O)CC3=CC=C(C=C3)O)CC4=CC=CC5=CC=CC=C54)C(=O)NCC6=CC=CC=C6 | ||
| Standard InChIKey | HQWTUOLCGKIECB-XZWHSSHBSA-N | ||
| Standard InChI | InChI=1S/C33H32N4O4/c38-27-15-13-23(14-16-27)19-29-32(40)35(21-26-11-6-10-25-9-4-5-12-28(25)26)22-30-36(18-17-31(39)37(29)30)33(41)34-20-24-7-2-1-3-8-24/h1-16,29-30,38H,17-22H2,(H,34,41)/t29-,30+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

ICG-001 Dilution Calculator

ICG-001 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.8227 mL | 9.1136 mL | 18.2272 mL | 36.4544 mL | 45.5681 mL |
| 5 mM | 0.3645 mL | 1.8227 mL | 3.6454 mL | 7.2909 mL | 9.1136 mL |
| 10 mM | 0.1823 mL | 0.9114 mL | 1.8227 mL | 3.6454 mL | 4.5568 mL |
| 50 mM | 0.0365 mL | 0.1823 mL | 0.3645 mL | 0.7291 mL | 0.9114 mL |
| 100 mM | 0.0182 mL | 0.0911 mL | 0.1823 mL | 0.3645 mL | 0.4557 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 3,29-O-Dibenzoyloxykarounidiol
Catalog No.:BCN9863
CAS No.:389122-01-4
- Somnifericin
Catalog No.:BCN9862
CAS No.:173693-57-7
- Solanidine
Catalog No.:BCN9861
CAS No.:80-78-4
- Sphenanlignan
Catalog No.:BCN9860
CAS No.:866347-36-6
- Vanillic acid glucoside
Catalog No.:BCN9859
CAS No.:32142-31-7
- alpha-Peltatin
Catalog No.:BCN9858
CAS No.:568-53-6
- 5,6-Benzoflavone
Catalog No.:BCN9857
CAS No.:6051-87-2
- Quasipanaxatriol
Catalog No.:BCN9856
CAS No.:171903-78-9
- n-Tridecane
Catalog No.:BCN9855
CAS No.:629-50-5
- 3-Hydroxybenzoic acid
Catalog No.:BCN9854
CAS No.:99-06-9
- 2-Benzal-4-hydroxyacetophenone
Catalog No.:BCN9853
CAS No.:2657-25-2
- Colchiceine
Catalog No.:BCN9852
CAS No.:477-27-0
- 4-(4-Methoxyphenyl)-2-butanone
Catalog No.:BCN9865
CAS No.:104-20-1
- Tropic acid
Catalog No.:BCN9866
CAS No.:529-64-6
- 1,2-Dimethoxybenzene
Catalog No.:BCN9867
CAS No.:91-16-7
- 15-Methoxy-16-oxo-15,16H-strictic acid
Catalog No.:BCN9868
CAS No.:1356388-38-9
- Datiscin
Catalog No.:BCN9869
CAS No.:16310-92-2
- Cannabiscitrin
Catalog No.:BCN9870
CAS No.:520-14-9
- Lappaconine
Catalog No.:BCN9871
CAS No.:23943-93-3
- Condurango glycoside A
Catalog No.:BCN9872
CAS No.:11051-90-4
- 2-(4-Hydroxybenzal)acetophenone
Catalog No.:BCN9873
CAS No.:20426-12-4
- Norharman
Catalog No.:BCN9874
CAS No.:244-63-3
- Syringetin
Catalog No.:BCN9875
CAS No.:4423-37-4
- 4-Hydroxyisophthalic acid
Catalog No.:BCN9876
CAS No.:636-46-4
WNT4 secreted by tumor tissues promotes tumor progression in colorectal cancer by activation of the Wnt/beta-catenin signalling pathway.[Pubmed:33222684]
J Exp Clin Cancer Res. 2020 Nov 23;39(1):251.
BACKGROUND: Wingless and Int-related protein (Wnt) ligands are aberrantly expressed in patients with colorectal cancer (CRC). However, the aberrant level of Wnt ligands in serum have not been explored. Here, we aimed to identify the levels of WNT4 in serum and explored its oncogenic role in CRC. METHODS: The Oncomine database was used to analyze the relationship between WNT4 and the prognosis of CRC. ELISA was performed to measure WNT4 levels in serum and conditioned medium from fresh CRC tissues and adjacent normal tissues. Western blot and immunohistochemistry were carried out to measure the expression of WNT4 in human CRC tissues and adjacent normal tissues. The migration and invasion of CRC cells were determined by trans-well assay, and the effects of WNT4 on CRC invasion and metastasis in vivo were verified by tumor xenograft in nude mice. Cancer-associated fibroblasts (CAFs) and angiogenesis in subcutaneous nodules were detected by immunofluorescence (IF). In addition, the suspended spheres formation and tube formation assay were performed to explore the effects of WNT4 on CAFs and angiogenesis respectively. RESULTS: WNT4 was significantly upregulated in serum of CRC patients, and CRC tissues were identified as an important source of elevated WNT4 levels in CRC patients. Interestingly, elevated levels of WNT4 in serum were downregulated after tumor resection. Furthermore, we found that WNT4 contributed to epithelial-to-mesenchymal transition (EMT) and activated fibroblasts by activating the WNT4/beta-catenin pathway in vitro and in vivo. Moreover, angiogenesis was induced via the WNT4/beta-catenin/Ang2 pathway. Those effects could be reversed by ICG-001, a beta-catenin/TCF inhibitor. CONCLUSION: Our findings indicated that serum levels of WNT4 may be a potential biomarker for CRC. WNT4 secreted by colorectal cancer tissues promote the progression of CRC by inducing EMT, activate fibroblasts and promote angiogenesis through the canonical Wnt/beta-catenin signalling pathway.
CBP-mediated Wnt3a/beta-catenin signaling promotes cervical oncogenesis initiated by Piwil2.[Pubmed:33190089]
Neoplasia. 2020 Nov 12;23(1):1-11.
Our previous work demonstrated that Piwil2 reactivated by the human papillomavirus oncoproteins E6 and E7 may reprogram somatic cells into tumor-initiating cells (TICs), which contribute to cervical neoplasia lesions. Maintaining the stemness of TICs is critical for the progression of cervical lesions. Here, we determined that canonical Wnt signaling was aberrantly activated in HaCaT cells transfected with lentivirus expressing Piwil2 and in cervical lesion specimens of low-grade squamous intraepithelial lesion, high-grade squamous intraepithelial lesion, and invasive carcinoma. Blocking the beta-catenin and CREB binding protein interaction with ICG-001 significantly downregulated the reprogramming factors c-Myc, Nanog, Oct4, Sox2, and Klf4, thus leading to cell differentiation and preventing tumorigenicity in Piwil2-overexpressing HaCaT cells. Similarly, Piwil2 also critically regulated the canonical Wnt signaling pathway in cervical cancer. We further demonstrated that ICG-001 increased cisplatin sensitivity and significantly suppressed tumor growth of cervical cancer alone or in combination with cisplatin both in vitro and in vivo. The beta-catenin/ CREB binding protein-mediated transcription activated by Piwil2 is essential for the maintenance of TICs, therefore contributing to the progression of cervical oncogenesis.
ALK-1 to ALK-5 ratio dictated by the Akt1-beta-catenin pathway regulates TGFbeta-induced endothelial-to-mesenchymal transition.[Pubmed:33157202]
Gene. 2020 Nov 4:145293.
Endothelial-to-mesenchymal transition (EndMT) indispensable in embryogenesis also occurs in several human pathologies. Although transforming growth factor-beta (TGFbeta) has been demonstrated to induce EndMT, the type-I receptors (ALK-1 and ALK-5) responsible for TGFbeta-induced EndMT is unclear. In the current study, we investigated the role of the Akt1 pathway in ALK1 and ALK5 expression regulation in response to TGFbeta1 and TGFbeta2 in human microvascular endothelial cells (HMECs). Whereas treatment with TGFbeta1 and TGFbeta2 or Akt1 gene silencing promoted EndMT accompanied by increased ALK5 expression and reduced ALK1 expression accompanied by increased expression of N-cadherin and reduced expression of eNOS in HMECs, treatment with ALK-5 inhibitor (SB431542) blunted these effects. Importantly, the inhibitor of beta-catenin (ICG-001) suppressed TGFbeta1- and TGFbeta2-induced ALK5 expression in both normal and Akt1 deficient HMECs indicating the integral role of Akt1-beta-catenin pathway in the regulation of ALK5 expression promoting EndMT.
FTO demethylates m6A modifications in HOXB13 mRNA and promotes endometrial cancer metastasis by activating the WNT signalling pathway.[Pubmed:33103587]
RNA Biol. 2020 Nov 5:1-14.
Although many studies have confirmed the relationship between obesity and endometrial cancer (EC), the molecular mechanism between obesity and EC progression has not been elucidated. Overexpression of fat mass and the obesity associated protein FTO leads to weight gain, although recently it has been discovered that FTO can serve as a demethylase which erases N6-methyladenosine (m6A) modification and regulates the metabolization of mRNAs. In this study, we found high expression of FTO in metastatic EC and that this action promote both metastasis and invasion in vivo and in vitro. Mechanistically, FTO can catalyse demethylation modification in 3'UTR region of HOXB13 mRNA, thereby abolishing m6A modification recognition with the YTHDF2 protein. Decreasing HOXB13 mRNA decay and increasing HOXB13 protein expression was accompanied by WNT signalling pathway activation and the expression of downstream proteins, leading to tumour metastasis and invasion. We also found the WNT signalling pathway inhibitor ICG-001 can block HOXB13 gene-induced tumour metastasis, therefore ICG-001 may be a promising molecular intervention. This study provides insight into the relationship between obesity and the pathogenesis of endometrial cancer while highlighting future areas of research.
Effects of Wnt signaling on epithelial to mesenchymal transition in chronic rhinosinusitis with nasal polyp.[Pubmed:33023995]
Thorax. 2020 Nov;75(11):982-993.
BACKGROUND: Epithelial to mesenchymal transition (EMT) is associated with the pathophysiology of chronic rhinosinusitis with nasal polyp (CRSwNP). Wnt signaling is causative for EMT, whereas the mechanism in CRSwNP is not fully understood. OBJECTIVE: We sought to evaluate the role of Wnt signaling in EMT of CRSwNP using a murine nasal polyp (NP) model and human tissues. METHODS: Inflammatory markers and EMT-related molecules were evaluated in NP models using adenomatosis polyposis coli (Apc)(Min/+) mice with activated Wnt signaling and NP models treated with Wnt signaling inhibitor, indocyanine green-001 (ICG-001). EMT markers and Wnt signaling-associated mediators were analysed using human sinonasal tissues from control subjects and CRSwNP patients. RESULTS: Apc(Min/+) mice-induced NPs exhibited more frequent polypoid lesions and upregulation of Wnt-related molecules, including nuclear beta-catenin, WNT3A and cyclin D1. Markers of EMT were significantly overexpressed in the Apc(Min/+) NP mice (p<0.001 for E-cadherin and alpha-smooth muscle actin), and interleukin (IL)-17A(+) cells and neutrophilic infiltration were increased in Apc(Min/+) NP mice (p<0.001). Inhibition of Wnt signaling via ICG-001 resulted in significantly decreased nasal polypoid lesions (p<0.001), EMT-related markers (p=0.019 for E-cadherin and p=0.002 for vimentin) and the mRNA levels of IL-4 (p<0.001) and IL-17A (p=0.004) compared with the positive control group. Finally, nuclear beta-catenin (p=0.042) was significantly increased compared with the control, and the expression levels of Wnt ligands and receptors were upregulated in human NP tissues (p=0.045 for WNT3A and p=0.042 for FZD2), suggesting increased Wnt signaling and EMT in CRSwNP. CONCLUSION: Wnt signaling may contribute to the pathogenesis of NPs through EMT. Therefore, inhibition of Wnt signaling may be a potential therapeutic strategy for patients with CRSwNP.
Combined treatment with CBP and BET inhibitors reverses inadvertent activation of detrimental super enhancer programs in DIPG cells.[Pubmed:32826850]
Cell Death Dis. 2020 Aug 21;11(8):673.
Diffuse intrinsic pontine gliomas (DIPG) are the most aggressive brain tumors in children with 5-year survival rates of only 2%. About 85% of all DIPG are characterized by a lysine-to-methionine substitution in histone 3, which leads to global H3K27 hypomethylation accompanied by H3K27 hyperacetylation. Hyperacetylation in DIPG favors the action of the Bromodomain and Extra-Terminal (BET) protein BRD4, and leads to the reprogramming of the enhancer landscape contributing to the activation of DIPG super enhancer-driven oncogenes. The activity of the acetyltransferase CREB-binding protein (CBP) is enhanced by BRD4 and associated with acetylation of nucleosomes at super enhancers (SE). In addition, CBP contributes to transcriptional activation through its function as a scaffold and protein bridge. Monotherapy with either a CBP (ICG-001) or BET inhibitor (JQ1) led to the reduction of tumor-related characteristics. Interestingly, combined treatment induced strong cytotoxic effects in H3.3K27M-mutated DIPG cell lines. RNA sequencing and chromatin immunoprecipitation revealed that these effects were caused by the inactivation of DIPG SE-controlled tumor-related genes. However, single treatment with ICG-001 or JQ1, respectively, led to activation of a subgroup of detrimental super enhancers. Combinatorial treatment reversed the inadvertent activation of these super enhancers and rescued the effect of ICG-001 and JQ1 single treatment on enhancer-driven oncogenes in H3K27M-mutated DIPG, but not in H3 wild-type pedHGG cells. In conclusion, combinatorial treatment with CBP and BET inhibitors is highly efficient in H3K27M-mutant DIPG due to reversal of inadvertent activation of detrimental SE programs in comparison with monotherapy.
AGE receptor 1 silencing enhances advanced oxidative protein product-induced epithelial-to-mesenchymal transition of human kidney proximal tubular epithelial cells via RAGE activation.[Pubmed:32819586]
Biochem Biophys Res Commun. 2020 Sep 3;529(4):1201-1208.
Advanced oxidative protein products (AOPPs) are novel uremic toxins whose concentrations continuously increases in patients with chronic kidney disease (CKD). Epithelial-to-mesenchymal transition (EMT) of tubular cells is the main mechanism underlying CKD pathogenesis. Studies have shown that AOPPs can induce EMT and promote renal fibrosis. However, the mechanism through which AOPPs induce tubular cell-EMT is poorly understood. In this study, we aimed to clarify the mechanisms underlying AOPP-induced EMT in human kidney proximal tubular (HKC-8) epithelial cells. Small molecule inhibitor, CRISPR-Cas9 knockout technology, siRNA knockdown technology, western blot, and reverse transcription-quantitative polymerase chain reaction were applied to investigate the mechanisms underlying AOPP-induced EMT in HKC-8 cells. AOPP treatment was found to significantly induce EMT, as evidenced by increased alpha-smooth muscle actin (alpha-SMA) and decreased E-cadherin levels, and upregulated Wnt1, beta-catenin, Tcf4, and Gsk-3beta expression. Conversely, blockade of Wnt/beta-catenin signaling using small molecule inhibitor ICG-001 hindered AOPP-induced EMT. Moreover, knockout of receptor of advanced glycation end-products (RAGE) reversed these aforementioned effects, whereas AGE receptor 1 (AGER1)-specific siRNA transfection enhanced them. Taken together, these data suggested that AOPPs could induce HKC-8 cell EMT by activating the RAGE/Wnt/beta-catenin signaling pathway and AGER1 could restore EMT by antagonizing the role of RAGE. These results may provide a new theoretical basis for EMT and help identify new therapeutic targets for suppressing CKD progression.
Effective Reconstruction of Functional Urethra Promoted With ICG-001 Delivery Using Core-Shell Collagen/Poly(Llactide-co-caprolactone) [P(LLA-CL)] Nanoyarn-Based Scaffold: A Study in Dog Model.[Pubmed:32754582]
Front Bioeng Biotechnol. 2020 Jul 10;8:774.
Hypospadias and urethral stricture are common urological diseases which seriously affect voiding function and life quality of the patients, yet current clinical treatments often result in unsatisfactory clinical outcome with frequent complications. In vitro experiments confirmed that ICG-001 (a well-established Wnt signaling inhibitor) could effectively suppress fibroblast proliferation and fibrotic protein expression. In this study, we applied a novel drug-delivering nanoyarn scaffold in urethroplasty in dog model, which continuously delivers ICG-001 during tissue reconstruction, and could effectively promote urethral recovery and resume fully functional urethra within 12 weeks. Such attempts are essential to the development of regenerative medicine for urological disorders and for broader clinical applications in human patients.
Role of miR-96/EVI1/miR-449a Axis in the Nasopharyngeal Carcinoma Cell Migration and Tumor Sphere Formation.[Pubmed:32752071]
Int J Mol Sci. 2020 Jul 31;21(15). pii: ijms21155495.
The Wnt signaling pathway is one of the major signaling pathways used by cancer stem cells (CSC). Ecotropic Viral Integration Site 1 (EVI1) has recently been shown to regulate oncogenic development of tumor cells by interacting with multiple signaling pathways, including the Wnt signaling. In the present study, we found that the Wnt modulator ICG-001 could inhibit the expression of EVI1 in nasopharyngeal carcinoma (NPC) cells. Results from loss-of-function and gain-of-function studies revealed that EVI1 expression positively regulated both NPC cell migration and growth of CSC-enriched tumor spheres. Subsequent studies indicated ICG-001 inhibited EVI1 expression via upregulated expression of miR-96. Results from EVI1 3'UTR luciferase reporter assay confirmed that EVI1 is a direct target of miR-96. Further mechanistic studies revealed that ICG-001, overexpression of miR-96, or knockdown of EVI1 expression could restore the expression of miR-449a. The suppressive effect of miR-449a on the cell migration and tumor sphere formation was confirmed in NPC cells. Taken together, the miR-96/EVI1/miR-449a axis is a novel pathway involved in ICG-001-mediated inhibition of NPC cell migration and growth of the tumor spheres.
The CREB-binding protein inhibitor ICG-001: a promising therapeutic strategy in sporadic meningioma with NF2 mutations.[Pubmed:32642722]
Neurooncol Adv. 2020 Feb 22;2(1):vdz055.
Background: Meningiomas with Neurofibromin 2 gene mutations (NF2-mutant meningiomas) account for ~40% of the sporadic meningiomas. However, there is still no effective drug treatment for the disease. Methods: Expression profile of Merlin protein was explored through immunohistochemistry in a meningioma patient cohort (n = 346). A 20-agent library covering a wide range of meningioma relevant targets was tested using meningioma cell lines IOMM-Lee (NF2 wildtype) and CH157-MN (NF2 deficient). Therapeutic effects and biological mechanisms of the identified compound, ICG-001, in NF2-mutant meningiomas were further characterized in vitro and in patient-derived xenograft (PDX) models. Results: Low Merlin expression was associated with meningioma proliferation and poor clinical outcomes in a large patient series. ICG-001, a cAMP-responsive element binding (CREB)-binding protein (CBP) inhibitor, selectively suppressed tumor growth of cells with low Merlin expression. Besides, ICG-001 mediated CH157-MN and IOMM-Lee growth inhibition primarily through robust induction of the G1 cell-cycle arrest. Treatment with ICG-001 alone significantly reduced the growth of NF2-mutant xenografts in mice, as well. We also provide further evidence that ICG-001 inhibits proliferation of NF2-mutant meningioma cells at least partly through attenuating the FOXM1-mediated Wnt/beta-catenin signaling. Conclusions: This study highlights the importance of ligand-mediated Wnt/beta-catenin signaling as well as its drugable potency in NF2-mutant meningioma.
Targeting the CBP/beta-Catenin Interaction to Suppress Activation of Cancer-Promoting Pancreatic Stellate Cells.[Pubmed:32516943]
Cancers (Basel). 2020 Jun 5;12(6). pii: cancers12061476.
BACKGROUND: Although cyclic AMP-response element binding protein-binding protein (CBP)/beta-catenin signaling is known to promote proliferation and fibrosis in various organ systems, its role in the activation of pancreatic stellate cells (PSCs), the key effector cells of desmoplasia in pancreatic cancer and fibrosis in chronic pancreatitis, is largely unknown. METHODS: To investigate the role of the CBP/beta-catenin signaling pathway in the activation of PSCs, we have treated mouse and human PSCs with the small molecule specific CBP/beta-catenin antagonist ICG-001 and examined the effects of treatment on parameters of activation. RESULTS: We report for the first time that CBP/beta-catenin antagonism suppresses activation of PSCs as evidenced by their decreased proliferation, down-regulation of "activation" markers, e.g., alpha-smooth muscle actin (alpha-SMA/Acta2), collagen type I alpha 1 (Col1a1), Prolyl 4-hydroxylase, and Survivin, up-regulation of peroxisome proliferator activated receptor gamma (Ppar-gamma) which is associated with quiescence, and reduced migration; additionally, CBP/beta-catenin antagonism also suppresses PSC-induced migration of cancer cells. CONCLUSION: CBP/beta-catenin antagonism represents a novel therapeutic strategy for suppressing PSC activation and may be effective at countering PSC promotion of pancreatic cancer.
Targeting JMJD3 histone demethylase mediates cardiac fibrosis and cardiac function following myocardial infarction.[Pubmed:32513540]
Biochem Biophys Res Commun. 2020 Aug 6;528(4):671-677.
Myocardial fibrosis is the pathological consequence of injury-induced fibroblastto-myofibroblast transition, resulting in increased stiffness and diminished cardiac function. Histone modification has been shown to play an important role in the pathogenesis of cardiac fibrosis. Here, we identified H3K27me3 demethylase JMJD3/KDM6B promotes cardiac fibrosis via regulation of fibrogenic pathways. Using neonatal rat cardiac fibroblasts (NRCF), we show that the expression of endogenous JMJD3 is induced by angiotensin II (Ang II), while the principle extracellular matrix (ECM) such as fibronectin, CTGF, collagen I and III are increased. We find that JMJD3 inhibition markedly enhances the suppressive mark (H3K27me3) at the beta (beta)-catenin promoter in activated cardiac fibroblasts, and then substantially decreases expression of fibrogenic gene. Both inhibition of beta-catenin-mediated transcription with ICG-001 and genetic loss of beta-catenin can prevent Ang II-induced ECM deposition. Most importantly, in vivo inhibition of JMJD3 rescues myocardial ischemia-induced cardiac fibrosis and cardiac dysfunction. Collectively, our findings are the first to report a novel role of histone demethylase JMJD3 in the pro-fibrotic cardiac fibroblast phenotype, pharmacological targeting of JMJD3 might represent a promising therapeutic approach for the treatment of human cardiac fibrosis and other fibrotic diseases.
Enhanced Generation of Induced Cardiomyocytes Using a Small-Molecule Cocktail to Overcome Barriers to Cardiac Cellular Reprogramming.[Pubmed:32500803]
J Am Heart Assoc. 2020 Jun 16;9(12):e015686.
Background Given known inefficiencies in reprogramming of fibroblasts into mature induced cardiomyocytes (iCMs), we sought to identify small molecules that would overcome these barriers to cardiac cell transdifferentiation. Methods and Results We screened alternative combinations of compounds known to impact cell reprogramming using morphologic and functional cell differentiation assays in vitro. After screening 6 putative reprogramming factors, we found that a combination of the histone deacetylase inhibitor sodium butyrate, the WNT inhibitor ICG-001, and the cardiac growth regulator retinoic acid (RA) maximally enhanced iCM generation from primary rat cardiac fibroblasts when combined with administration of the cardiodifferentiating transcription factors Gata4, Mef2C, and Tbx5 (GMT) compared with GMT administration alone (23+/-1.5% versus 3.3+/-0.2%; P<0.0001). Expression of the cardiac markers cardiac troponin T, Myh6, and Nkx2.5 was upregulated as early as 10 days after GMT-sodium butyrate, ICG-001, and RA treatment. Human iCM generation was likewise enhanced when administration of the human cardiac reprogramming factors GMT, Hand2, and Myocardin plus miR-590 was combined with sodium butyrate, ICG-001, and RA compared with GMT, Hand2, and Myocardin plus miR-590 treatment alone (25+/-1.3% versus 5.7+/-0.4%; P<0.0001). Rat and human iCMs also more frequently demonstrated spontaneous beating in coculture with neonatal cardiomyocytes with the addition of sodium butyrate, ICG-001, and RA to transcription factor cocktails compared with transcription factor treatment alone. Conclusions The combined administration of histone deacetylase and WNT inhibitors with RA enhances rat and human iCM generation induced by transcription factor administration alone. These findings suggest opportunities for improved translational approaches for cardiac regeneration.


