FlavonolCAS# 577-85-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

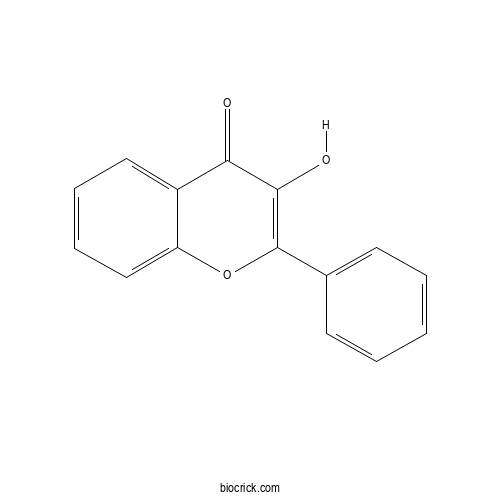
| Cas No. | 577-85-5 | SDF | Download SDF |
| PubChem ID | 11349 | Appearance | Powder |
| Formula | C15H10O3 | M.Wt | 238.2 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 3-hydroxy-2-phenylchromen-4-one | ||
| SMILES | C1=CC=C(C=C1)C2=C(C(=O)C3=CC=CC=C3O2)O | ||
| Standard InChIKey | HVQAJTFOCKOKIN-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C15H10O3/c16-13-11-8-4-5-9-12(11)18-15(14(13)17)10-6-2-1-3-7-10/h1-9,17H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Flavonol has antioxidant activity. | |||||

Flavonol Dilution Calculator

Flavonol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.1982 mL | 20.9908 mL | 41.9815 mL | 83.9631 mL | 104.9538 mL |
| 5 mM | 0.8396 mL | 4.1982 mL | 8.3963 mL | 16.7926 mL | 20.9908 mL |
| 10 mM | 0.4198 mL | 2.0991 mL | 4.1982 mL | 8.3963 mL | 10.4954 mL |
| 50 mM | 0.084 mL | 0.4198 mL | 0.8396 mL | 1.6793 mL | 2.0991 mL |
| 100 mM | 0.042 mL | 0.2099 mL | 0.4198 mL | 0.8396 mL | 1.0495 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- alpha-Ionone
Catalog No.:BCN9849
CAS No.:127-41-3
- Gardenin A
Catalog No.:BCN9848
CAS No.:21187-73-5
- Urushiol (15:3)
Catalog No.:BCN9847
CAS No.:83543-37-7
- Vitexin 7-glucoside
Catalog No.:BCN9846
CAS No.:35109-95-6
- 9-Hydroxy-O-senecioyl-8,9-dihydrooroselol
Catalog No.:BCN9845
CAS No.:31456-63-0
- Tryptanthrine
Catalog No.:BCN9844
CAS No.:13220-57-0
- Eugenol benzoate
Catalog No.:BCN9843
CAS No.:531-26-0
- Butyl acetate
Catalog No.:BCN9842
CAS No.:123-86-4
- Bletilol B
Catalog No.:BCN9841
CAS No.:147235-17-4
- Sutherlandioside D
Catalog No.:BCN9840
CAS No.:1055329-49-1
- Pilocarpine
Catalog No.:BCN9839
CAS No.:92-13-7
- 5-Methoxyflavone
Catalog No.:BCN9838
CAS No.:42079-78-7
- Helveticoside
Catalog No.:BCN9851
CAS No.:630-64-8
- Colchiceine
Catalog No.:BCN9852
CAS No.:477-27-0
- 2-Benzal-4-hydroxyacetophenone
Catalog No.:BCN9853
CAS No.:2657-25-2
- 3-Hydroxybenzoic acid
Catalog No.:BCN9854
CAS No.:99-06-9
- n-Tridecane
Catalog No.:BCN9855
CAS No.:629-50-5
- Quasipanaxatriol
Catalog No.:BCN9856
CAS No.:171903-78-9
- 5,6-Benzoflavone
Catalog No.:BCN9857
CAS No.:6051-87-2
- alpha-Peltatin
Catalog No.:BCN9858
CAS No.:568-53-6
- Vanillic acid glucoside
Catalog No.:BCN9859
CAS No.:32142-31-7
- Sphenanlignan
Catalog No.:BCN9860
CAS No.:866347-36-6
- Solanidine
Catalog No.:BCN9861
CAS No.:80-78-4
- Somnifericin
Catalog No.:BCN9862
CAS No.:173693-57-7
The Influence of Solvent, Host, and Phenological Stage on the Yield, Chemical Composition, and Antidiabetic and Antioxidant Properties of Phragmanthera capitata (Sprengel) S. Balle.[Pubmed:33293990]
Evid Based Complement Alternat Med. 2020 Nov 18;2020:6284925.
Phragmanthera capitata was reported to possess many biological properties making it a good candidate for the formulation of a phytomedicine with multiple effects. In this work, we studied some factors likely to modify these therapeutic properties with the aim to contribute to its standardization as an improved traditional medicine. P. capitata parasitizing Persea americana, Psidium guajava, and Podocarpus mannii were harvested at three phenological stages (vegetative, flowering, and fruiting stages). The extracts were prepared by maceration in n-hexane, ethyl acetate, ethanol, methanol, and distilled water. The total phenolic, flavonoid, Flavonol, and tannin contents were measured using appropriate methods. The antioxidant potential of extracts was investigated using TAC, DPPH scavenging, and FRAP methods. The alpha-amylase and alpha-glucosidase inhibitory activities of extracts were determined using enzymatic methods. The ethyl acetate extracts with the best phenolic content were subjected to HPLC analysis. The extraction yields were higher with methanol. The ethyl acetate extract of P. capitata harvested from P. guajava showed a stable HPLC profile during the development of the plant, while extracts from the plant collected from P. americana and P. mannii showed both qualitative and quantitative variations according to phonological stages of the plant. The inhibition of alpha-amylase was more pronounced for P. capitata harvested from P. guajava, decreasing during flowering and fruiting, while inhibition of alpha-glucosidase was not influenced by the phenological stage and the host of the plant. The alpha-amylase inhibitors were better extracted by ethyl acetate and those of alpha-glucosidase by ethanol or methanol. The phenolic contents and antioxidant properties of the extracts were influenced by the phenological stage of P. capitata and its hosts. These results suggest that it is preferable to harvest P. capitata during flowering or during fruiting stages on any host. None of the used solvents permitted an optimal extraction of active principles form P. capitata, suggesting that the mixture of solvents must be considered in further studies.
Characterization of extractable phenolic profile of common bean seeds (Phaseolus vulgaris L.) in a Spanish diversity panel.[Pubmed:33292961]
Food Res Int. 2020 Dec;138(Pt A):109713.
Phenolic compounds are important bioactive compounds in common bean (Phaseolus vulgaris L.). The aim of this work was the characterization of extractable phenolic profile (corresponding to 12 hydroxycinnamic acids and derivatives, 13 anthocyanins and 15 Flavonols) in a bean diversity panel constituted by 220 lines, all grown under the same environmental conditions. Hydroxycinnamic derivatives were detected in all samples, while anthocyanins and Flavonols were not detected in samples with completely white seed coats. In general, lines with black seeds showed higher contents of anthocyanins, followed by some red-seeded lines, while notable levels of Flavonols were detected in market classes, including those with yellow, pink, and cream seed coats. However, a clear relationship between phenolic composition and seed phenotype could not be established, indicating the great influence of the genotype. This wide variability in the phenolic profiles analyzed is of particular interest for further breeding trials and the selection of varieties on the basis of this group of compounds.
Metabolomics and gene expression analysis reveal the accumulation patterns of phenylpropanoids and flavonoids in different colored-grain wheats (Triticum aestivum L.).[Pubmed:33292960]
Food Res Int. 2020 Dec;138(Pt A):109711.
Colored-grain wheats have received increasing attention owing to their high nutritional values. In this study, we compared the metabolomes of four pigmented wheat cultivars with conventional yellow wheat using an ultra-performance liquid chromatography-electrospray ionization-tandem mass spectrometry (UPLC-ESI-MS/MS)-based metabolomics approach. A total of 711 metabolites were identified, and considerable differences were observed in the flavonoid and phenylpropanoid metabolites among five samples by orthogonal signal correction and partial least squares-discriminant analysis (OPLS-DA) analysis. These differential metabolites were significantly enriched in the "anthocyanin biosynthesis", "flavones and Flavonols biosynthesis", and "flavonoids biosynthesis" pathways. Furthermore, the expression of 9 structural genes and 2 regulatory genes involved in flavonoid biosynthesis pathway were investigated by quantitative real-time PCR (qRT-PCR). Results suggested that blue, red, purple, and black wheat cultivars showed higher transcription levels of structural and regulatory genes in the flavonoid pathway than that of conventional yellow wheat, possibly accounting for the abundant anthocyanin accumulation in the grains of these four cultivars. This study laid a foundation for understanding the accumulation of flavonoids and coloration mechanisms in colored-grain wheats, and provides a theoretical basis for their sufficient utilization.
Characterization of Bioactive Compounds of Opuntia ficus-indica (L.) Mill. Seeds from Spanish Cultivars.[Pubmed:33291779]
Molecules. 2020 Dec 4;25(23). pii: molecules25235734.
Opuntia ficus-indica (L.) Mill. is the Cactaceae plant with the greatest economic relevance in the world. It can be used for medicinal purposes, animal nutrition, production of biofuels and phytoremediation of soils. Due to its high content of bioactive compounds, the prickly pear has antioxidant, antimicrobial and anticancer properties. The aim of this study was to determine the polyphenolic, fatty acid and amino acid profile and characterize the antioxidant capacity of seeds of seven Spanish prickly pear cultivars. A total of 21 metabolites, mainly phenolic acids and Flavonols, were identified using ultraperformance liquid chromatography photodiode detector quadrupole/time-of-flight mass spectrometry (UPLC-PDA-Q/TOF-MS). Significant differences were found in the phenolic concentrations of the investigated varieties. The highest amount of phenolic compounds (266.67 mg/kg dry matter) were found in the "Nopal espinoso" variety, while the "Fresa" variety was characterized by the lowest content (34.07 mg/kg DM) of these compounds. In vitro antioxidant capacity was positively correlated with the amount of polyphenols. The amino acid composition of protein contained in prickly pear seeds was influenced by the variety. Glutamic acid was the predominant amino acid followed by arginine, aspartic acid and leucine, independent of prickly pear variety. Overall, 13 different fatty acids were identified and assessed in prickly pear seeds. The dominant fatty acid was linoleic acid, with content varying between 57.72% "Nopal ovalado" and 63.11% "Nopal espinoso".
Discovery of hCES2A inhibitors from Glycyrrhiza inflata via combination of docking-based virtual screening and fluorescence-based inhibition assays.[Pubmed:33291124]
Food Funct. 2020 Dec 8.
Human carboxylesterase 2 (hCES2A) is a key target to ameliorate the intestinal toxicity triggered by irinotecan that causes severe diarrhea in 50%-80% of patients receiving this anticancer agent. Herbal medicines are frequently used for the prevention and treatment of the intestinal toxicity of irinotecan, but it is very hard to find strong hCES2A inhibitors from herbal medicines in an efficient way. Herein, an integrated strategy via combination of chemical profiling, docking-based virtual screening and fluorescence-based high-throughput inhibitor screening assays was utilized. Following the screening of a total of 73 herbal products, licorice (the dried root of Glycyrrhiza species) was found with the most potent hCES2A inhibition activity. Further investigation revealed that the chalcones and several Flavonols in licorice displayed strong hCES2A inhibition activities, while isoliquiritigenin, echinatin, naringenin, gancaonin I and glycycoumarin exhibited moderate inhibition of hCES2A. Inhibition kinetic analysis demonstrated that licochalcone A, licochalcone C, licochalcone D and isolicoFlavonol potently inhibited hCES2A-mediated fluorescein diacetate hydrolysis in a reversible and mixed inhibition manner, with Ki values less than 1.0 muM. Further investigations demonstrated that licochalcone C, the most potent hCES2A inhibitor identified from licorice, dose-dependently inhibited intracellular hCES2A in living HepG2 cells. In summary, this study proposed an integrated strategy to find hCES2A inhibitors from herbal medicines, and our findings suggested that the chalcones and isolicoFlavonol in licorice were the key ingredients responsible for hCES2A inhibition, which would be very helpful to develop new herbal remedies or drugs for ameliorating hCES2A-associated drug toxicity.
JA-Responsive Transcription Factor SmMYB97 Promotes Phenolic Acid and Tanshinone Accumulation in Salvia miltiorrhiza.[Pubmed:33284615]
J Agric Food Chem. 2020 Dec 7.
Phenolic acids and tanshinones are active principles in Salvia miltiorrhiza Bunge administered for cardiovascular and cerebrovascular diseases. Jasmonic acid (JA) promotes secondary metabolite accumulation, but the regulatory mechanism is unknown in S. miltiorrhiza. We identified and characterized the JA-responsive gene SmMYB97. Multiple sequence alignment and phylogenetic tree analyses showed that SmMYB97 was clustered with AtMYB11, AtMYB12, and ZmP1 in the subgroup S7 regulating Flavonol biosynthesis. SmMYB97 was highly expressed in S. miltiorrhiza leaves and induced by methyl jasmonate (MeJA). SmMYB97 was localized in the nucleus and had strong transcriptional activation activity. SmMYB97 overexpression increased phenolic acid and tanshinone biosynthesis and upregulated the genes implicated in these processes. Yeast one-hybrid and transient transcriptional activity assays disclosed that SmMYB97 binds the PAL1, TAT1, CPS1, and KSL1 promoter regions. SmJAZ8 interacts with SmMYB97 and downregulates the genes that it controls. This study partially clarified the regulatory network of MeJA-mediated secondary metabolite biosynthesis in S. miltiorrhiza.
A comparative UPLC-Q-Orbitrap-MS untargeted metabolomics investigation of different parts of Clausena lansium (Lour.) Skeels.[Pubmed:33282233]
Food Sci Nutr. 2020 Oct 3;8(11):5811-5822.
In this study, the non-targeted large-scale plant metabolomics (UPLC-Q-Orbitrap-MS) was performed for the comparison of chemical profiling of the leaves, barks, flowers, peels, pulps, and seeds of Clausena lansium (Lour.) Skeels (called "wampee"). A total of 364 metabolites were identified, and 62 potential biomarkers were selected by the multivariate statistical analysis. Hierarchical cluster analysis suggested that the selected biomarkers were significant differential metabolites among various parts of wampee. Metabolic pathway analysis showed a significant enrichment of the "Flavone and Flavonol synthesis" and "Isoquinoline alkaloid biosynthesis" pathway. This study provides important information for the isolation and identification of functional components from different tissues of wampee and the metabolic biosynthesis pathway elucidation in detail.
The Mastic Tree (Pistacia lentiscus L.) Leaves as Source of BACs: Effect of Growing Location, Phenological Stage and Extraction Solvent on Phenolic Content.[Pubmed:33281486]
Food Technol Biotechnol. 2020 Sep;58(3):303-314.
Research background: Mastic tree (Pistacia lentiscus L.) of the Anacardiaceae family is an evergreen shrub from Mediterranean countries where it is used in traditional medicine. Analysis of P. lentiscus leaf, stem, fruit and root extracts showed high concentrations of principal groups of secondary metabolites (flavonoids, phenolic acids and tannins), suggesting the plant possesses great biological potential. Therefore, the aim of this research is to evaluate the impact of environmental parameters and the extraction solvent type on the concentration of phenols in mastic tree leaf extracts grown at four different locations along the Adriatic coast (Barbariga, Lun, Hvar and Vela Luka) during three phenological stages (early flowering, early fruiting and late fruiting). Experimental approach: Since mastic tree plant has phenolic compounds with different structures and chemical properties, ethanolic and methanolic leaf extracts were analysed using high-performance liquid chromatography (HPLC) coupled with UV/Vis PDA detector. Phenolic compounds were identified by comparing the retention times and spectral data with those of standards at 280 and 340 nm. Results and conclusions: In all samples, phenolic acids and Flavonol glycosides were quantified, while catechin was quantified only in methanolic extracts. The 5-O-galloylquinic acid was determined as a predominant phenolic compound in all samples followed by monogalloyl glucose, 3,5-di-O-galloylquinic acid, 3,4,5-tri-O-galloylquinic acid and gallic acid, respectively. Myricetin-3-O-rhamnoside was found to be the predominant Flavonol glycoside followed by myricetin-3-O-glucoside, myricetin-3-O-glucuronide, quercetin-3-O-rhamnoside and derivative of Flavonol glycoside. The mass concentration of these compounds significantly varied during different phenological stages, at different growing locations and used extraction solvents. The highest phenolic mass concentration was determined in the samples harvested at Hvar growing location and extracted in 80% methanol. The highest total phenolic acid mass concentration was obtained in the samples harvested during the flowering phenological stage and the highest total flavonoid mass concentration in the samples harvested during the early fruiting stage. Novelty and scientific contribution: The obtained data provide a better understanding of the P. lentiscus species phenolic concentration, which can lead to further investigations regarding the valorisation of mastic tree leaves as pharmaceutical products or as food products with added value.
Flavonoids and caffeoylquinic acids in Chrysanthemum morifolium Ramat flowers: A potentially rich source of bioactive compounds.[Pubmed:33280963]
Food Chem. 2020 Nov 27:128733.
Varieties of chrysanthemums are among the world's most valuable edible ornamental crops. However, the availability and relationship between the bio-chemicals of chrysanthemums and their morphological variations remain unclear. We developed liquid chromatography mass spectrometry to construct a spectral tag library to identify and quantify chemicals of 7 caffeoylquinic acids, 21 flavones and Flavonols, 4 carotenoids, and 13 other compounds in 27 cultivars and representative tea of Chrysanthemum morifolium. A correlation analysis found that more acacetin 7-O-galactoside (23) resulted in lighter colored flowers and less acacetin (43) and kaempferol (44) was associated with yellow flowers. Hot-H2O extraction of C. morifolium tea showed that most flavonoids and caffeoylquinic acids dissolved out at 30 min, with 20.977 and 8.958 mg/g GW indicated that C. morifolium, which is used in food and tea, is rich in flavonoids and carotenoids. The results improve our understanding of flavonoid biosynthesis and the mechanisms responsible for flower color.
Effects of Supplementary Blue and UV-A LED Lights on Morphology and Phytochemicals of Brassicaceae Baby-Leaves.[Pubmed:33276420]
Molecules. 2020 Dec 2;25(23). pii: molecules25235678.
Brassicaceae baby-leaves are good source of functional phytochemicals. To investigate how Chinese kale and pak-choi baby-leaves in response to different wavebands of blue (430 nm and 465 nm) and UV-A (380 nm and 400 nm) LED, the plant growth, glucosinolates, antioxidants, and minerals were determined. Both agronomy traits and phytochemical contents were significantly affected. Blue and UV-A light played a predominant role in increasing the plant biomass and morphology, as well as the contents of antioxidant compounds (vitamin C, vitamin E, phenolics, and individual Flavonols), the antioxidant activity (DPPH and FRAP), and the total glucosinolates accumulation. In particular, four light wavebands significantly decreased the content of progoitrin, while 400 nm UV-A light and 430 nm blue light were efficient in elevating the contents of sinigrin and glucobrassicin in Chinese kale. Meanwhile, 400 nm UV-A light was able to increase the contents of glucoraphanin, sinigrin, and glucobrassicin in pak-choi. From the global view of heatmap, blue lights were more efficient in increasing the yield and phytochemical levels of two baby-leaves.
Pretreatment of IEC-6 cells with quercetin and myricetin resists the indomethacin-induced barrier dysfunction via attenuating the calcium-mediated JNK/Src activation.[Pubmed:33276066]
Food Chem Toxicol. 2020 Dec 1;147:111896.
This study investigated the protective effect of two Flavonols quercetin and myricetin on barrier function of rat intestinal epithelial (IEC-6) cells with indomethacin injury. When the cells were pretreated with the heated or unheated Flavonols of 2.5-10 mumol/L for 24-48 h and then injured by 300 mumol/L indomethacin for 24 h, they showed reduced lactate dehydrogenase release (LDH) but increased cell viability; however, the Flavonols of 20 mumol/L exerted a little effect to increase cell viability or decrease LDH release. Cell pretreatment with 5 mumol/L Flavonols also resisted cell barrier dysfunction by increasing transepithelial resistance, reducing paracellular permeability, and promoting mRNA and protein expression of three tight junction proteins zonula occluden-1, occludin, and claudin-1. Although indomethacin injury increased intracellular Ca(2+) concentration ([Ca(2+)]i) and consequently caused JNK/Src activation, the Flavonols could decrease [Ca(2+)]i and attenuate the calcium-mediated JNK/Src activation. Quercetin with less hydroxyl groups was more efficient than myricetin to resist barrier dysfunction, while the unheated Flavonols were more active than the heated counterparts to perform this effect. It is thus proposed that quercetin and myricetin could resist barrier dysfunction of the intestine once injured by indomethacin, but heat treatment of Flavonols had a negative impact on barrier-protective function of Flavonols.
New phenylpropanoid-substituted and benzyl-substituted flavonols from Alangium chinense.[Pubmed:33276012]
Fitoterapia. 2020 Dec 1:104792.
Two previously undescribed Flavonols with phenylpropanoid or benzyl substitution, named alangsine A (1), and alangsine B (2), together with four known compounds (3-6) were isolated from the leaves of Alangium chinense. Alangsine A was a racemic mixture, which was further separated into two enantiomers via high-performance liquid chromatography on a chiral column. The absolute configurations of the enantiomer pairs were deduced from the circular dichroism (CD) spectra. The activity of the isolated compounds towards neuronal excitability was examined.
Isolation and Characterisation of Two Quercetin Glycosides with Potent Anti-Reactive Oxygen Species (ROS) Activity and an Olean-12-en Triterpene Glycoside from the Fruit of Abelmoschus esculentus (Malvaceae Juss.).[Pubmed:33274571]
Chem Biodivers. 2020 Dec 3.
Abelmoschus esculentus (okra) is used in the traditional treatment of cancer, hyperlipidaemia and hyperglycaemia. We, therefore, investigated its composition and potential cytotoxic or antioxidant properties that might underlie its phytotherapeutic applications. Its methanolic fruit extract yielded compounds 1, 2 and 3, identified through NMR, UV and MS analyses as Olean-12-en-3-O-beta-D-glucopyranoside, isoquercitrin (quercetin glycoside) and 5,7,3',4'-tetrahydroxy-Flavonol-3-O-[beta-D-glucopyranosyl-(1-->6)]-beta-D-glucop yranoside (quercetin diglycoside), respectively. Following 48 h exposure, oleane glycoside was mildly toxic to the HeLa and the MRC5-SV2 cancer cells, isoquercitrin was not toxic except at 100 microg/ml in HeLa, and quercetin diglycoside elicited no toxicity. In a 2',7'-dichlorofluorescein diacetate (DCFDA) assay of intracellular levels of reactive oxygen species (ROS), hydrogen peroxide increased ROS levels, an effect not affected by oleane glycoside but protected against by isoquercitrin and quercetin diglycoside, with IC50 values, respectively, of 2.7 +/- 0.5 microg/ml and 1.9 +/- 0.2 microg/ml (3 h post-treatment) and 2.0 +/- 0.8 microg/ml and 1.5 +/- 0.4 microg/ml (24 h post-treatment.) This is the first report of an oleanene skeleton triterpenoid in this plant. The work provides some insight into why the plant is included in remedies for cancers, cardiovascular complications and diabetes, and reveals it as a potential source of novel therapeutics.
Physiological and transcriptomic analyses reveal the roles of secondary metabolism in the adaptive responses of Stylosanthes to manganese toxicity.[Pubmed:33272205]
BMC Genomics. 2020 Dec 3;21(1):861.
BACKGROUND: As a heavy metal, manganese (Mn) can be toxic to plants. Stylo (Stylosanthes) is an important tropical legume that exhibits tolerance to high levels of Mn. However, little is known about the adaptive responses of stylo to Mn toxicity. Thus, this study integrated both physiological and transcriptomic analyses of stylo subjected to Mn toxicity. RESULTS: Results showed that excess Mn treatments increased malondialdehyde (MDA) levels in leaves of stylo, resulting in the reduction of leaf chlorophyll concentrations and plant dry weight. In contrast, the activities of enzymes, such as peroxidase (POD), phenylalanine ammonia-lyase (PAL) and polyphenol oxidase (PPO), were significantly increased in stylo leaves upon treatment with increasing Mn levels, particularly Mn levels greater than 400 muM. Transcriptome analysis revealed 2471 up-regulated and 1623 down-regulated genes in stylo leaves subjected to Mn toxicity. Among them, a set of excess Mn up-regulated genes, such as genes encoding PAL, cinnamyl-alcohol dehydrogenases (CADs), chalcone isomerase (CHI), chalcone synthase (CHS) and Flavonol synthase (FLS), were enriched in secondary metabolic processes based on gene ontology (GO) analysis. Numerous genes associated with transcription factors (TFs), such as genes belonging to the C2H2 zinc finger transcription factor, WRKY and MYB families, were also regulated by Mn in stylo leaves. Furthermore, the C2H2 and MYB transcription factors were predicted to be involved in the transcriptional regulation of genes that participate in secondary metabolism in stylo during Mn exposure. Interestingly, the activation of secondary metabolism-related genes probably resulted in increased levels of secondary metabolites, including total phenols, flavonoids, tannins and anthocyanidins. CONCLUSIONS: Taken together, this study reveals the roles of secondary metabolism in the adaptive responses of stylo to Mn toxicity, which is probably regulated by specific transcription factors.


