Gardenin ACAS# 21187-73-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 21187-73-5 | SDF | Download SDF |
| PubChem ID | 261859 | Appearance | Powder |
| Formula | C21H22O9 | M.Wt | 418.4 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
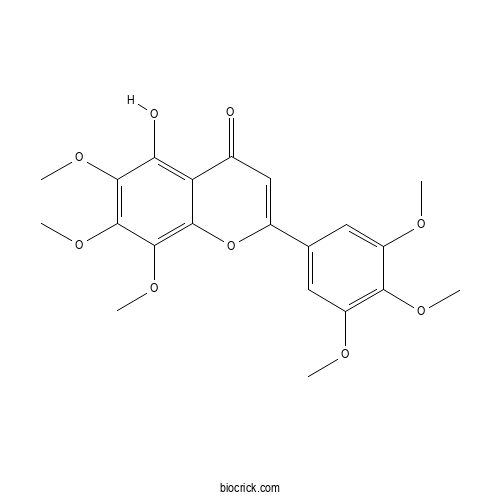
| Chemical Name | 5-hydroxy-6,7,8-trimethoxy-2-(3,4,5-trimethoxyphenyl)chromen-4-one | ||
| SMILES | COC1=CC(=CC(=C1OC)OC)C2=CC(=O)C3=C(C(=C(C(=C3O2)OC)OC)OC)O | ||
| Standard InChIKey | MQBFFYQCZCKSBX-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C21H22O9/c1-24-13-7-10(8-14(25-2)17(13)26-3)12-9-11(22)15-16(23)19(27-4)21(29-6)20(28-5)18(15)30-12/h7-9,23H,1-6H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Gardenin A has antihyperlipidemic and hepatoprotective effects , it also promotes neuritogenesis through the activation of MAPK/ERK-, PKC-, and PKA-dependent, but not TrkA-dependent, CREB signaling pathways in PC12 cells. | |||||

Gardenin A Dilution Calculator

Gardenin A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3901 mL | 11.9503 mL | 23.9006 mL | 47.8011 mL | 59.7514 mL |
| 5 mM | 0.478 mL | 2.3901 mL | 4.7801 mL | 9.5602 mL | 11.9503 mL |
| 10 mM | 0.239 mL | 1.195 mL | 2.3901 mL | 4.7801 mL | 5.9751 mL |
| 50 mM | 0.0478 mL | 0.239 mL | 0.478 mL | 0.956 mL | 1.195 mL |
| 100 mM | 0.0239 mL | 0.1195 mL | 0.239 mL | 0.478 mL | 0.5975 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Urushiol (15:3)
Catalog No.:BCN9847
CAS No.:83543-37-7
- Vitexin 7-glucoside
Catalog No.:BCN9846
CAS No.:35109-95-6
- 9-Hydroxy-O-senecioyl-8,9-dihydrooroselol
Catalog No.:BCN9845
CAS No.:31456-63-0
- Tryptanthrine
Catalog No.:BCN9844
CAS No.:13220-57-0
- Eugenol benzoate
Catalog No.:BCN9843
CAS No.:531-26-0
- Butyl acetate
Catalog No.:BCN9842
CAS No.:123-86-4
- Bletilol B
Catalog No.:BCN9841
CAS No.:147235-17-4
- Sutherlandioside D
Catalog No.:BCN9840
CAS No.:1055329-49-1
- Pilocarpine
Catalog No.:BCN9839
CAS No.:92-13-7
- 5-Methoxyflavone
Catalog No.:BCN9838
CAS No.:42079-78-7
- Isobutyl acetate
Catalog No.:BCN9837
CAS No.:110-19-0
- Urushiol (15:2)
Catalog No.:BCN9836
CAS No.:83258-37-1
- alpha-Ionone
Catalog No.:BCN9849
CAS No.:127-41-3
- Flavonol
Catalog No.:BCN9850
CAS No.:577-85-5
- Helveticoside
Catalog No.:BCN9851
CAS No.:630-64-8
- Colchiceine
Catalog No.:BCN9852
CAS No.:477-27-0
- 2-Benzal-4-hydroxyacetophenone
Catalog No.:BCN9853
CAS No.:2657-25-2
- 3-Hydroxybenzoic acid
Catalog No.:BCN9854
CAS No.:99-06-9
- n-Tridecane
Catalog No.:BCN9855
CAS No.:629-50-5
- Quasipanaxatriol
Catalog No.:BCN9856
CAS No.:171903-78-9
- 5,6-Benzoflavone
Catalog No.:BCN9857
CAS No.:6051-87-2
- alpha-Peltatin
Catalog No.:BCN9858
CAS No.:568-53-6
- Vanillic acid glucoside
Catalog No.:BCN9859
CAS No.:32142-31-7
- Sphenanlignan
Catalog No.:BCN9860
CAS No.:866347-36-6
Major flavonoids from Psiadia punctulata produce vasodilation via activation of endothelial dependent NO signaling.[Pubmed:32382447]
J Adv Res. 2020 Jan 3;24:273-279.
Vasodilators are important pharmacologic agents for managing and/or treating hypertension. Medicinal plants are considered as valuable source of bioactive compounds. We used a bioguided approach to isolate, identify, and investigate the possible vasodilation activities and mechanism(s) of the prepared methanol extract from aerial parts of Psiadia punctulata (MAPP), its bioactive fraction and active compounds. Vascular effects of MAPP were studied using isolated artery technique in the presence or absence of specific candidate pathways inhibitors, and found to produce a significant vasodilation of phenylephrine preconstricted rat aortae. The bioactive chloroform fraction yielded five methoxylated flavonoids: umuhengerin (1), Gardenin A (2), gardenin B (3), luteolin-3',4' -dimethyl ether (4), and 5,3'-dihydroxy-6,7,4',5'-tetramethoxyflavone (5). Metabolites 1, 4, and 5 produced a significant vasodilation. Removal of the endothelium significantly inhibited MAPP vasodilation. Nitric oxide synthase inhibition and not prostacycline inhibition or K(+) channel blocking, was found to cause the observed vasodilation inhibition. Both guanylate cyclase and adenylate cyclase inhibitions markedly inhibited MAPP vasodilation. In conclusion MAPP possesses vasodilation activities that is mediated through endothelial nitric oxide pathway, calcium dependent endothelial nitric oxide synthase activation, and interference with the depolarization process through calcium channel blocking activity.
Evaluation of the neuropharmacological effects of Gardenin A in mice.[Pubmed:32181517]
Drug Dev Res. 2020 Aug;81(5):600-608.
This work describes the neuropharmacological (sedative, anxiolytic, antidepressant, and anticonvulsant) actions of Gardenin A (GA) (0.1-25 mg/kg p.o.), a flavonoid found in medicinal plants. The sedative effects of GA were assessed with the pentobarbital-induced sleep test. The anxiolytic actions of GA were evaluated with the elevated plus-maze, the light-dark box test, the exploratory cylinder assay, and the open field test. Motor coordination was evaluated with the rotarod test and the open field test. The antidepressant-like actions of GA were evaluated with the tail suspension test and forced swimming test. The mechanisms of the anxiolytic-like and antidepressant-like effects of GA were assessed using inhibitors of neurotransmission pathways. The anticonvulsant activity of GA was evaluated with the strychnine-induced seizure test. The sedative effects of GA were evident only at a dose of 25 mg/kg, which increased the duration of sleep but did not alter sleep onset. GA showed anxiolytic-like actions with activity comparable to that of clonazepam in all experimental tests. The GABAA receptor antagonist bicuculline reversed the anxiolytic-like effects of GA. Furthermore, GA showed significant antidepressant-like actions in both models with activity comparable to that of fluoxetine. Yohimbine, an alpha2-adrenoceptor blocker, inhibited the antidepressant-like actions of GA. In addition, GA (1-10 mg/kg) did not affect locomotor coordination in mice and delayed the onset of convulsions. These findings suggest that GA induces anxiolytic-like effects and has anticonvulsant actions by the possible involvement of the GABAergic system. The antidepressant-like actions of GA may be mediated by noradrenergic neurotransmission.
Antihyperlipidemic and hepatoprotective effects of Gardenin A in cellular and high fat diet fed rodent models.[Pubmed:28351695]
Chem Biol Interact. 2017 May 1;269:9-17.
The gum of Gardenia resinifera Roth., is one of the important drugs used in the Indian system of medicine and a source of unique polymethoxylated flavones. This study was aimed to evaluate the antihyperlipidemic and anti-NAFLD effects of Gardenin A (Gar-A) from G. resinifera gum using in vitro and in vivo models. Gar-A was isolated from G. resinifera gum and was identified on the basis of the physical and spectral data. Toxicity of Gar-A to HepG2 cells was evaluated using MTT assay. The ability of Gar-A to reduce steatosis was assessed using oleate-palmitate induced HepG2 cell lines by estimating the lipid levels by ORO staining and by estimating the intracellular triglyceride content. Effect of Gar-A on amelioration of lipotoxicity was measured by estimating the LDH levels. The doses for in vivo experiments were fixed by Irwin test, between 50 and 100 mg/kg concentrations, through oral route. The acute antihyperlipidemic effect of Gar-A was assessed in Triton WR-1339 induced hyperlipidemic animals. The chronic antihyperlipidemic and anti-NAFLD effects of Gar-A were evaluated in HFD fed rats. In vitro experiments with HepG2 cell line indicated that the cells treated with Gar-A did not show any significant reduction in the viability up to 70 mug/mL concentration. Steatotic HepG2 cells treated with Gar-A showed a significant reduction in lipid accumulation at 2.5-10 mug/mL concentrations. In triton induced hyperlipidemic rats, the treatment significantly reduced the lipid levels at the synthesis phase. The treatment with Gar-A to the HFD fed animals significantly lowered the steatosis and transaminase levels. The other biochemical parameters such as TC, TG, LDL-c, ALP and ACP were also decreased significantly. Treatment with Gar-A significantly lowered the hyperlipidemia and fat accumulation in the liver; detailed molecular investigations are necessary to establish the antihyperlipidemic and hepatoprotective potentials of Gar-A.
Identification of metabolites of gardenin A in rats by combination of high-performance liquid chromatography with linear ion trap-Orbitrap mass spectrometer based on multiple data processing techniques.[Pubmed:25041995]
Biomed Chromatogr. 2015 Mar;29(3):379-87.
Gardenin A is one of the less abundant hydroxylated polymethoxyflavonoids (OH-PMFs) in nature, and has many potential significant health benefits. In the present study, an efficient strategy was established using high-performance liquid chromatography coupled with linear ion trap-Orbitrap mass spectrometer to profile the in vivo metabolic fate of Gardenin A in rat plasma and various tissues. First, an online LC-MS(n) data acquisition method was developed to trace all the probable metabolites. Second, a combination of offline data processing methods including extracted ion chromatography and multiple mass defect filters was employed to screen the common and uncommon metabolites from the background noise and endogenous components. Finally, structures of the metabolites were elucidated based on an accurate mass measurement, the diagnostic product ions of PMFs, and relevant drug biotransformation knowledge. Based on the proposed strategy, a total of 26 metabolites were observed and characterized. The results indicate that some biotransformations, such as methylation, demethoxylation, demethylation, glucuronide conjugation, sulfate conjugation and their composite reactions, have been discovered for OH-PMFs. Moreover, some diagnostic biotransformation pathways are summarized. Overall, this study gives us a first insight into the in vivo metabolism of Gardenin A. The study also provides a practical strategy for rapidly screening and identifying metabolites, which can be widely applied for the other biotransformations.
Neurotrophic action of 5-hydroxylated polymethoxyflavones: 5-demethylnobiletin and gardenin A stimulate neuritogenesis in PC12 cells.[Pubmed:24003765]
J Agric Food Chem. 2013 Oct 2;61(39):9453-63.
Polymethoxyflavones (PMFs) exhibit a broad spectrum of biological properties, including anticancer, antiatherogenic, and neuroprotective effects. The aim of this study is to investigate the neurotrophic effects of 5-demethylnobiletin, a hydroxylated PMF found in citrus plants, and Gardenin A, a synthetic PMF analogue, on neurite outgrowth and neuronal differentiation in PC12 cells. The results of this study showed that 5-demethylnobiletin and Gardenin A (10-20 muM) potently induce neurite outgrowth in PC12 cells, accompanied by the expression of neuronal differentiation and synapse formation marker proteins, growth-associated protein-43 (GAP-43), and synaptophysin. We observed that the addition of K252a, a TrKA antagonist, significantly inhibited NGF-induced neurite outgrowth in PC12 cells, but 5-demethylnobiletin- or Gardenin A-induced neurite outgrowth was not affected. Treatment with 5-demethylnobiletin and Gardenin A markedly induced the phosphorylation of both cyclic AMP response element-binding protein (CREB) and CRE-mediated transcription, which was suppressed through the administration of the inhibitor 2-naphthol AS-E phosphate (KG-501) or using CREB siRNA. Furthermore, our results showed that MAPK/ERK kinase 1/2 (MEK1/2), protein kinase A (PKA), and protein kinase C (PKC) inhibitors blocked the CRE transcription activity and neurite outgrowth induced through 5-demethylnobiletin or Gardenin A. Consistently, increased ERK phosphorylation and PKA and PKC activities were observed in PC12 cells treated with 5-demethylnobiletin or Gardenin A. These results reveal for the first time that 5-demethylnobiletin and Gardenin A promote neuritogenesis through the activation of MAPK/ERK-, PKC-, and PKA-dependent, but not TrkA-dependent, CREB signaling pathways in PC12 cells.


