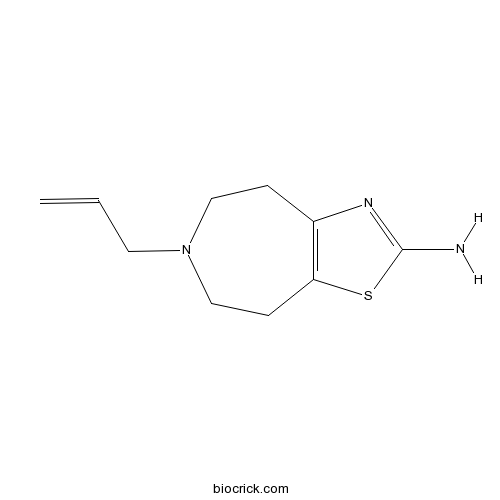
TalipexoleCAS# 101626-70-4 |
- Sitagliptin phosphate monohydrate
Catalog No.:BCC2111
CAS No.:654671-77-9
- Alogliptin (SYR-322)
Catalog No.:BCC2113
CAS No.:850649-61-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 101626-70-4 | SDF | Download SDF |
| PubChem ID | 5374 | Appearance | Powder |
| Formula | C10H15N3S | M.Wt | 209.31 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in DMSO | ||
| Chemical Name | 6-prop-2-enyl-4,5,7,8-tetrahydro-[1,3]thiazolo[4,5-d]azepin-2-amine | ||
| SMILES | C=CCN1CCC2=C(CC1)SC(=N2)N | ||
| Standard InChIKey | DHSSDEDRBUKTQY-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C10H15N3S/c1-2-5-13-6-3-8-9(4-7-13)14-10(11)12-8/h2H,1,3-7H2,(H2,11,12) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Talipexole Dilution Calculator

Talipexole Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.7776 mL | 23.888 mL | 47.776 mL | 95.5521 mL | 119.4401 mL |
| 5 mM | 0.9555 mL | 4.7776 mL | 9.5552 mL | 19.1104 mL | 23.888 mL |
| 10 mM | 0.4778 mL | 2.3888 mL | 4.7776 mL | 9.5552 mL | 11.944 mL |
| 50 mM | 0.0956 mL | 0.4778 mL | 0.9555 mL | 1.911 mL | 2.3888 mL |
| 100 mM | 0.0478 mL | 0.2389 mL | 0.4778 mL | 0.9555 mL | 1.1944 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Trenbolone acetate
Catalog No.:BCC9184
CAS No.:10161-34-9
- Trenbolone
Catalog No.:BCC9183
CAS No.:10161-33-8
- N-Acetyltryptamine
Catalog No.:BCC6618
CAS No.:1016-47-3
- 6-O-Nicotinoylbarbatin C
Catalog No.:BCN5828
CAS No.:1015776-92-7
- GSK 4716
Catalog No.:BCC7557
CAS No.:101574-65-6
- trans-2,3-Dihydro-3-ethoxyeuparin
Catalog No.:BCN6923
CAS No.:1015698-14-2
- Yadanzioside M
Catalog No.:BCN6712
CAS No.:101559-99-3
- Yadanzioside K
Catalog No.:BCN6714
CAS No.:101559-98-2
- Fmoc-D-Pro-OH
Catalog No.:BCC3540
CAS No.:101555-62-8
- Latifolin
Catalog No.:BCN7778
CAS No.:10154-42-4
- Methyl salvionolate A
Catalog No.:BCN3475
CAS No.:1015171-69-3
- 5''-O-Syringoylkelampayoside A
Catalog No.:BCN4798
CAS No.:1014974-98-1
- Kadsuracoccinic acid A
Catalog No.:BCN5829
CAS No.:1016260-22-2
- Diacetylpiquerol A
Catalog No.:BCC8935
CAS No.:130466-34-1
- Sodium Monofluorophosphate
Catalog No.:BCC4722
CAS No.:10163-15-2
- Drevogenin A
Catalog No.:BCN4740
CAS No.:10163-83-4
- Cyclo(Hpro-Leu)
Catalog No.:BCN2430
CAS No.:1016899-93-6
- Cyclo(Phe-Hpro)
Catalog No.:BCN2425
CAS No.:1016899-94-7
- Barpisoflavone A
Catalog No.:BCN4739
CAS No.:101691-27-4
- Olivil monoacetate
Catalog No.:BCN4738
CAS No.:1016974-78-9
- Sulfocostunolide A
Catalog No.:BCN5830
CAS No.:1016983-51-9
- 6-O-p-Hydroxybenzoylaucubin
Catalog No.:BCN5831
CAS No.:1016987-87-3
- Myzodendrone
Catalog No.:BCN7257
CAS No.:101705-37-7
- (S)-(+)-4-Benzyl-3-propionyl-2-oxazolidinone
Catalog No.:BCC8399
CAS No.:101711-78-8
Release and aggregation of cytochrome c and alpha-synuclein are inhibited by the antiparkinsonian drugs, talipexole and pramipexole.[Pubmed:11301060]
Eur J Pharmacol. 2001 Apr 6;417(1-2):59-67.
Recently, it has been shown that release of cytochrome c from the mitochondria to the cytosol is required for activation of the caspase-3-dependent cascade in apoptosis, and also for alpha-synuclein aggregation. In the present study, we examined the effects of Talipexole and pramipexole on the release of cytochrome c and alpha-synuclein, their aggregations, and activation of caspases. Treatment of human neuroblastoma SH-SY5Y cells with 1-methyl-4-phenylpyridinium (MPP(+), 1 mM) induced the first event, which was the release of cytochrome c from the organellar fraction to the cytosolic fraction, then came the DNA fragmentation, and caused the last event, which was the accumulation of alpha-synuclein protein in the cytosolic fraction. Talipexole and pramipexole at low concentration (0.1-1 mM) significantly inhibited the accumulation of cytochrome c or alpha-synuclein in the cytosolic fraction. These drugs at high concentration (3-10 mM) inhibited in vitro aggregation of cytochrome c by hydrogen peroxide or that of alpha-synuclein by cytochrome c and hydrogen peroxide. In addition, in vitro activation of caspase-3 induced by cytochrome c and/or dATP was also inhibited by drugs at high concentration (5-10 mM). These results suggest that Talipexole and pramipexole may have protective effects against the neurodegeneration, which is induced by intracellular accumulation of cytochrome c and alpha-synuclein.
Pharmacokinetic profile of talipexole in healthy volunteers is not altered when it is co-administered with Madopar (co-beneldopa).[Pubmed:19646081]
J Clin Pharm Ther. 2009 Jun;34(3):345-54.
OBJECTIVES: To investigate the pharmacokinetics of Talipexole hydrochloride tablets and the potential influence of Madopar (benserazide and levodopa combination; co-beneldopa) tablets on Talipexole's pharmacokinetics when the two tablets are co-administered orally to healthy Chinese volunteers. METHODS: A sensitive liquid chromatography-tandem mass spectrometry (LC-MS/MS) method was developed to measure Talipexole concentration in human plasma in an open-label, randomized, two-way crossover, single-dose study, with 1-week washout period. Healthy Chinese volunteers were randomized to receive Talipexole tablets either alone or together with Madopar tablets by oral administration after an overnight fast. Serial blood samples were collected for a period of 36 h after the administration. Pharmacokinetic parameters C(max), t(max), t(1/2z), mean residence time (MRT), AUC(0-tau), AUC(0-infinity), CL(z)/F and V(z)/F were determined under the non-compartmental model. Pharmacokinetic values of Talipexole administered alone to the subjects were compared with those administered simultaneously with Madopar to determine whether or not the differences were statistically significant. RESULTS: The subjects experienced mild gastrointestinal irritation when Talipexole was administered alone as well as together with Madopar. For Talipexole hydrochloride, there were no significant differences in the pharmacokinetic values between the two administrations. No pharmacokinetic differences based on gender were observed either. CONCLUSION: A single oral dose of Madopar co-administered with Talipexole does not significantly change Talipexole's pharmacokinetics in human.
The neuroprotective effect of talipexole from paraquat-induced cell death in dopaminergic neuronal cells.[Pubmed:20673835]
Neurotoxicology. 2010 Dec;31(6):701-8.
Talipexole is a non-ergot dopamine (DA) agonist that has been used in the treatment of Parkinson's disease. In the present study, we examined the effect of Talipexole on paraquat (PQ)-induced N27 cell death and the intracellular pathways involved in this effect. Pretreatment of N27 cells with Talipexole (1mM) resulted in significant protection against paraquat-induced cell death. In N27 cells, Talipexole inhibited paraquat-induced apoptotic hallmarks such as cytochrome c release, caspase-3 activation, chromatin condensation and externalization of phosphatidilserine. Talipexole pretreatment prevents the reduction in the anti-apoptotic Bcl-x(L) protein and increases in the pro-apoptotic form of Bak and p-Bad, both induced by PQ. Finally, we also observed that Talipexole abrogates the activation of cell death pathways JNK1/2 and p38 produced by PQ, and increases the phosphorylated (active) forms of the pro-survival pathways ERK1/2 and Akt. These results reveal that Talipexole exerts a neuroprotective effect in a mesencephalic cell line exposed to the neurotoxin PQ, which is related to the etiology of Parkinson's disease.
Piribedil enhances frontocortical and hippocampal release of acetylcholine in freely moving rats by blockade of alpha 2A-adrenoceptors: a dialysis comparison to talipexole and quinelorane in the absence of acetylcholinesterase inhibitors.[Pubmed:12649387]
J Pharmacol Exp Ther. 2003 Apr;305(1):338-46.
In a dialysis procedure not requiring perfusate addition of acetylcholinesterase inhibitors to "boost" basal levels of acetylcholine (ACh), the influence of the antiparkinson agent piribedil upon levels of ACh in frontal cortex and dorsal hippocampus of freely moving rats was compared with those of other antiparkinson drugs and selective ligands at alpha(2)-adrenoceptors (ARs). Suggesting a tonic, inhibitory influence of alpha(2A)-ARs upon cholinergic transmission, the alpha(2)-AR agonist 5-bromo-6-[2-imidazolin-2-yl-amino]-quinoxaline tartrate (UK14,304), and the preferential alpha(2A)-AR agonist guanabenz reduced levels of ACh. They were elevated by the antagonists 2(2-methoxy-1,4 benzodioxan-2-yl)-2-imidazoline HCl (RX821002) and atipamezole and by the preferential alpha(2A)-AR antagonist 2-(2H-(1-methyl-1,3-dihydroisoindole)methyl)-4,5-dihydroimidazole (BRL44008). In contrast, trans-2,3,9,13b-tetrahydro-1,2-dimethyl-1H-dibenz[c,f]imidazo[1,5-a]azepine (BRL41992) and prazosin, preferential alpha(2B/2C)-AR antagonists, were inactive. The dopaminergic agonist and antiparkinson agent piribedil, which behaves as an antagonist at alpha(2)-ARs, dose dependently increased extracellular levels of ACh. This action was absent upon pretreatment with a maximally effective dose of RX821002. On the other hand, a further dopaminergic agonist and antiparkinson agent, Talipexole, which possesses agonist properties at alpha(2)-ARs, dose dependently reduced levels of ACh. This action was also blocked by RX821002. In contrast to piribedil and Talipexole, quinelorane, which interacts with dopaminergic receptors but not alpha(2)-ARs, failed to affect ACh levels. Finally, in analogy to the frontal cortex, piribedil likewise elicited a dose-dependent increase in extracellular levels of ACh in the dorsal hippocampus. In conclusion, in distinction to Talipexole and quinelorane, and reflecting its antagonist properties at alpha(2A)-ARs, piribedil reinforces cholinergic transmission in the frontal cortex and dorsal hippocampus of freely moving rats. These actions may be related to its facilitatory influence upon cognitive function.


