GSK 4716CAS# 101574-65-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 101574-65-6 | SDF | Download SDF |
| PubChem ID | 5331325 | Appearance | Powder |
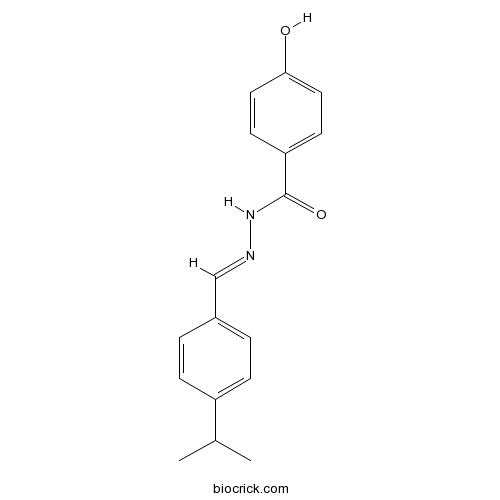
| Formula | C17H18N2O2 | M.Wt | 282.34 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | GW4716 | ||
| Solubility | DMSO : ≥ 100 mg/mL (354.18 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | 4-hydroxy-N-[(E)-(4-propan-2-ylphenyl)methylideneamino]benzamide | ||
| SMILES | CC(C)C1=CC=C(C=C1)C=NNC(=O)C2=CC=C(C=C2)O | ||
| Standard InChIKey | IKPPIUNQWSRCOZ-WOJGMQOQSA-N | ||
| Standard InChI | InChI=1S/C17H18N2O2/c1-12(2)14-5-3-13(4-6-14)11-18-19-17(21)15-7-9-16(20)10-8-15/h3-12,20H,1-2H3,(H,19,21)/b18-11+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective agonist at estrogen-related receptors ERRβ and ERRγ. Displays selectivity over ERRα and the classical estrogen receptors. |

GSK 4716 Dilution Calculator

GSK 4716 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.5418 mL | 17.7091 mL | 35.4183 mL | 70.8366 mL | 88.5457 mL |
| 5 mM | 0.7084 mL | 3.5418 mL | 7.0837 mL | 14.1673 mL | 17.7091 mL |
| 10 mM | 0.3542 mL | 1.7709 mL | 3.5418 mL | 7.0837 mL | 8.8546 mL |
| 50 mM | 0.0708 mL | 0.3542 mL | 0.7084 mL | 1.4167 mL | 1.7709 mL |
| 100 mM | 0.0354 mL | 0.1771 mL | 0.3542 mL | 0.7084 mL | 0.8855 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- trans-2,3-Dihydro-3-ethoxyeuparin
Catalog No.:BCN6923
CAS No.:1015698-14-2
- Yadanzioside M
Catalog No.:BCN6712
CAS No.:101559-99-3
- Yadanzioside K
Catalog No.:BCN6714
CAS No.:101559-98-2
- Fmoc-D-Pro-OH
Catalog No.:BCC3540
CAS No.:101555-62-8
- Latifolin
Catalog No.:BCN7778
CAS No.:10154-42-4
- Methyl salvionolate A
Catalog No.:BCN3475
CAS No.:1015171-69-3
- 5''-O-Syringoylkelampayoside A
Catalog No.:BCN4798
CAS No.:1014974-98-1
- GSK 0660
Catalog No.:BCC7688
CAS No.:1014691-61-2
- VTP-27999 2,2,2-trifluoroacetate
Catalog No.:BCC2049
CAS No.:1013937-63-7
- CP 376395 hydrochloride
Catalog No.:BCC7604
CAS No.:1013933-37-3
- Gadolinium chloride
Catalog No.:BCC7971
CAS No.:10138-52-0
- Formosanol
Catalog No.:BCN5826
CAS No.:101312-79-2
- 6-O-Nicotinoylbarbatin C
Catalog No.:BCN5828
CAS No.:1015776-92-7
- N-Acetyltryptamine
Catalog No.:BCC6618
CAS No.:1016-47-3
- Trenbolone
Catalog No.:BCC9183
CAS No.:10161-33-8
- Trenbolone acetate
Catalog No.:BCC9184
CAS No.:10161-34-9
- Talipexole
Catalog No.:BCC5250
CAS No.:101626-70-4
- Kadsuracoccinic acid A
Catalog No.:BCN5829
CAS No.:1016260-22-2
- Diacetylpiquerol A
Catalog No.:BCC8935
CAS No.:130466-34-1
- Sodium Monofluorophosphate
Catalog No.:BCC4722
CAS No.:10163-15-2
- Drevogenin A
Catalog No.:BCN4740
CAS No.:10163-83-4
- Cyclo(Hpro-Leu)
Catalog No.:BCN2430
CAS No.:1016899-93-6
- Cyclo(Phe-Hpro)
Catalog No.:BCN2425
CAS No.:1016899-94-7
- Barpisoflavone A
Catalog No.:BCN4739
CAS No.:101691-27-4
Estrogen-related receptors as emerging targets in cancer and metabolic disorders.[Pubmed:16515477]
Curr Top Med Chem. 2006;6(3):203-15.
While estrogen receptor (ER)-targeted therapeutics have clearly been a success in the treatment of breast cancer, the orphan estrogen-related receptors (ERRs) represent novel targets for future development. The ERRs, comprising ERRalpha, ERRbeta and ERRgamma, bind and regulate transcription via estrogen response elements (EREs) and extended ERE half-sites termed ERR response elements (ERREs), but do not bind endogenous estrogens. The emerging role of ERRalpha and ERRgamma in modulating estrogen responsiveness, substituting for ER activities, and serving as prognosticators in breast and other cancers is providing an impetus for the identification of compounds which target these proteins. Moreover, ERRalpha plays a role in energy homeostasis and will likely be targeted for the treatment of metabolic disorders. Multiple classes of synthetic ligands have already been identified. The phytoestrogens genistein, daidzein, biochanin A and 6,3'4'-tryhydroxyflavone have been reported as ERRalpha agonists. The phenolic acyl hydrazones GSK4716 and GSK9089 act as selective agonists of ERRbeta and ERRgamma. The organochlorine pesticides toxaphene and chlordane, and the synthetic compound XCT790 antagonize ERRalpha. The synthetic estrogen diethylstilbestrol antagonizes all three ERRs. The selective estrogen receptor modulators 4-hydroxytamoxifen and 4-hydroxytoremifene antagonize ERRgamma. The rational development of synthetic ligands for the ERRs may soon provide new agents to supplement the repertoire of antihormonal therapies to combat breast cancer. Moreover, expression of ERRs in other cancers and metabolic disorders may provide a targeted treatment strategy for these patients as well.
X-ray crystal structures of the estrogen-related receptor-gamma ligand binding domain in three functional states reveal the molecular basis of small molecule regulation.[Pubmed:16990259]
J Biol Chem. 2006 Dec 8;281(49):37773-81.
X-ray crystal structures of the ligand binding domain (LBD) of the estrogen-related receptor-gamma (ERRgamma) were determined that describe this receptor in three distinct states: unliganded, inverse agonist bound, and agonist bound. Two structures were solved for the unliganded state, the ERRgamma LBD alone, and in complex with a coregulator peptide representing a portion of receptor interacting protein 140 (RIP140). No significant differences were seen between these structures that both exhibited the conformation of ERRgamma seen in studies with other coactivators. Two structures were obtained describing the inverse agonist-bound state, the ERRgamma LBD with 4-hydroxytamoxifen (4-OHT), and the ERRgamma LBD with 4-OHT and a peptide representing a portion of the silencing mediator of retinoid and thyroid hormone action protein (SMRT). The 4-OHT structure was similar to other reported inverse agonist bound structures, showing reorientation of phenylalanine 435 and a displacement of the AF-2 helix relative to the unliganded structures with little other rearrangement occurring. No significant changes to the LBD appear to be induced by peptide binding with the addition of the SMRT peptide to the ERRgamma plus 4-OHT complex. The observed agonist-bound state contains the ERRgamma LBD, a ligand (GSK4716), and the RIP140 peptide and reveals an unexpected rearrangement of the phenol-binding residues. Thermal stability studies show that agonist binding leads to global stabilization of the ligand binding domain. In contrast to the conventional mechanism of nuclear receptor ligand activation, activation of ERRgamma by GSK4716 does not appear to involve a major rearrangement or significant stabilization of the C-terminal helix.
Identification and structure-activity relationship of phenolic acyl hydrazones as selective agonists for the estrogen-related orphan nuclear receptors ERRbeta and ERRgamma.[Pubmed:15857113]
J Med Chem. 2005 May 5;48(9):3107-9.
The first small molecule agonists of the estrogen-related receptors have been identified. GSK4716 (3) and GSK9089 (4) show binding to ERRgamma with remarkable selectivity over the classical estrogen receptors. Notably, in cell-based reporter assays, 3 mimics the protein ligand PGC-1alpha in activation of human ERRbeta and ERRgamma.


