GSK 0660CAS# 1014691-61-2 |
- Adefovir Dipivoxil
Catalog No.:BCC5025
CAS No.:142340-99-6
- Merimepodib
Catalog No.:BCC4128
CAS No.:198821-22-6
- Telbivudine
Catalog No.:BCC3862
CAS No.:3424-98-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1014691-61-2 | SDF | Download SDF |
| PubChem ID | 46233311 | Appearance | Powder |
| Formula | C19H18N2O5S2 | M.Wt | 418.49 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 49 mg/mL (117.09 mM) *"≥" means soluble, but saturation unknown. | ||
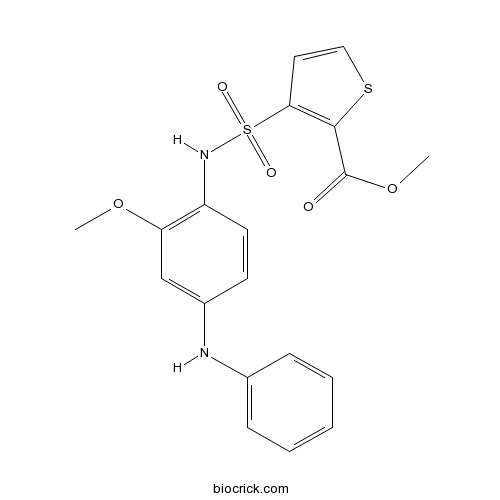
| Chemical Name | methyl 3-[(4-anilino-2-methoxyphenyl)sulfamoyl]thiophene-2-carboxylate | ||
| SMILES | COC1=C(C=CC(=C1)NC2=CC=CC=C2)NS(=O)(=O)C3=C(SC=C3)C(=O)OC | ||
| Standard InChIKey | NDFKBGWLUHKMFY-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C19H18N2O5S2/c1-25-16-12-14(20-13-6-4-3-5-7-13)8-9-15(16)21-28(23,24)17-10-11-27-18(17)19(22)26-2/h3-12,20-21H,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective PPARδ antagonist (IC50 values are 0.155, > 10 and ≥ 10 μM at PPARδ, PPARα and PPARγ respectively). Exhibits inverse agonist effects when administered by itself. |

GSK 0660 Dilution Calculator

GSK 0660 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3895 mL | 11.9477 mL | 23.8954 mL | 47.7909 mL | 59.7386 mL |
| 5 mM | 0.4779 mL | 2.3895 mL | 4.7791 mL | 9.5582 mL | 11.9477 mL |
| 10 mM | 0.239 mL | 1.1948 mL | 2.3895 mL | 4.7791 mL | 5.9739 mL |
| 50 mM | 0.0478 mL | 0.239 mL | 0.4779 mL | 0.9558 mL | 1.1948 mL |
| 100 mM | 0.0239 mL | 0.1195 mL | 0.239 mL | 0.4779 mL | 0.5974 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
GSK0660 is a potent antagonist of PPARβ and PPARδ, with IC50s of both 155 nM, and is nearly inactive on PPARα and PPARγ with IC50s of both >10 μM.
In Vitro:GSK0660 is a potent antagonist of PPARβ and PPARδ, with IC50s of both 155 nM, and is nearly inactive on PPARα and PPARγ with IC50s of both >10 μM. GSK0660 antagonizes 100% of the activity of PPARβ/δ with a pIC50 of 6.8. GSK0660 (100 nM) reduces CPT1a (a PPARβ/δ target gene) expression below the basal vehicle-treated level by approximately 50%, but shows no effect on PDK4 expression, which is also a PPARβ/δ target gene in skeletal muscle cells[1]. GSK0660 (0.5 μM) reduces the levels of AMPK and eNOS phosphorylation, and BMP-2, Runx-2 mRNA expression in MC3T3-E1 cells. GSK0660 (0.1 and 0.5 μM) reverses the bezafibrate-induced enchancement of ALP activity on d 7 in MC3T3-E1 cells[2]. GSK0660 (1 μM) markedly blocks GW501516-mediated attenuation of glutamate release, and the effect of GW501516 on ROS generation in BV-2 cells stimulated with LPS. Furthermore, GSK0660 significantly reduces inhibitory effect of GW501516 on the LPS-induced expression of gp91phox mRNA in BV-2 cells[3].
References:
[1]. Shearer BG, et al. Identification and characterization of a selective peroxisome proliferator-activated receptor beta/delta (NR1C2) antagonist. Mol Endocrinol. 2008 Feb;22(2):523-9. Epub 2007 Nov 1.
[2]. Zhong X, et al. Bezafibrate enhances proliferation and differentiation of osteoblastic MC3T3-E1 cells via AMPK and eNOS activation. Acta Pharmacol Sin. 2011 May;32(5):591-600.
[3]. Lee WJ, et al. Activation of PPARδ attenuates neurotoxicity by inhibiting lipopolysaccharide-triggered glutamate release in BV-2 microglial cells. J Cell Biochem. 2018 Feb 1.
- VTP-27999 2,2,2-trifluoroacetate
Catalog No.:BCC2049
CAS No.:1013937-63-7
- CP 376395 hydrochloride
Catalog No.:BCC7604
CAS No.:1013933-37-3
- Gadolinium chloride
Catalog No.:BCC7971
CAS No.:10138-52-0
- Formosanol
Catalog No.:BCN5826
CAS No.:101312-79-2
- PF-04691502
Catalog No.:BCC3837
CAS No.:1013101-36-4
- Zacopride hydrochloride
Catalog No.:BCC7178
CAS No.:101303-98-4
- Noreugenin
Catalog No.:BCN5827
CAS No.:1013-69-0
- PETCM
Catalog No.:BCC2360
CAS No.:10129-56-3
- 11-Chloro-2,3-dihydro-2-methyl-1H- dibenz[2,3:6,7]oxepino[4,5-c]pyrrol-1-one
Catalog No.:BCC8431
CAS No.:1012884-46-6
- Phenserine
Catalog No.:BCC7529
CAS No.:101246-66-6
- Kushenol M
Catalog No.:BCN3310
CAS No.:101236-51-5
- Kushenol L
Catalog No.:BCN3309
CAS No.:101236-50-4
- 5''-O-Syringoylkelampayoside A
Catalog No.:BCN4798
CAS No.:1014974-98-1
- Methyl salvionolate A
Catalog No.:BCN3475
CAS No.:1015171-69-3
- Latifolin
Catalog No.:BCN7778
CAS No.:10154-42-4
- Fmoc-D-Pro-OH
Catalog No.:BCC3540
CAS No.:101555-62-8
- Yadanzioside K
Catalog No.:BCN6714
CAS No.:101559-98-2
- Yadanzioside M
Catalog No.:BCN6712
CAS No.:101559-99-3
- trans-2,3-Dihydro-3-ethoxyeuparin
Catalog No.:BCN6923
CAS No.:1015698-14-2
- GSK 4716
Catalog No.:BCC7557
CAS No.:101574-65-6
- 6-O-Nicotinoylbarbatin C
Catalog No.:BCN5828
CAS No.:1015776-92-7
- N-Acetyltryptamine
Catalog No.:BCC6618
CAS No.:1016-47-3
- Trenbolone
Catalog No.:BCC9183
CAS No.:10161-33-8
- Trenbolone acetate
Catalog No.:BCC9184
CAS No.:10161-34-9
Activation of Ras-ERK Signaling and GSK-3 by Amyloid Precursor Protein and Amyloid Beta Facilitates Neurodegeneration in Alzheimer's Disease.[Pubmed:28374012]
eNeuro. 2017 Mar 27;4(2). pii: eN-NWR-0149-16.
It is widely accepted that amyloid beta (Abeta) generated from amyloid precursor protein (APP) oligomerizes and fibrillizes to form neuritic plaques in Alzheimer's disease (AD), yet little is known about the contribution of APP to intracellular signaling events preceding AD pathogenesis. The data presented here demonstrate that APP expression and neuronal exposure to oligomeric Abeta42 enhance Ras/ERK signaling cascade and glycogen synthase kinase 3 (GSK-3) activation. We find that RNA interference (RNAi)-directed knockdown of APP in B103 rat neuroblastoma cells expressing APP inhibits Ras-ERK signaling and GSK-3 activation, indicating that APP acts upstream of these signal transduction events. Both ERK and GSK-3 are known to induce hyperphosphorylation of tau and APP at Thr668, and our findings suggest that aberrant signaling by APP facilitates these events. Supporting this notion, analysis of human AD brain samples showed increased expression of Ras, activation of GSK-3, and phosphorylation of APP and tau, which correlated with Abeta levels in the AD brains. Furthermore, treatment of primary rat neurons with Abeta recapitulated these events and showed enhanced Ras-ERK signaling, GSK-3 activation, upregulation of cyclin D1, and phosphorylation of APP and tau. The finding that Abeta induces Thr668 phosphorylation on APP, which enhances APP proteolysis and Abeta generation, denotes a vicious feedforward mechanism by which APP and Abeta promote tau hyperphosphorylation and neurodegeneration in AD. Based on these results, we hypothesize that aberrant proliferative signaling by APP plays a fundamental role in AD neurodegeneration and that inhibition of this would impede cell cycle deregulation and neurodegeneration observed in AD.
Transient Cerebral Ischemia Alters GSK-3beta and p-GSK-3beta Immunoreactivity in Pyramidal Neurons and Induces p-GSK-3beta Expression in Astrocytes in the Gerbil Hippocampal CA1 Area.[Pubmed:28349361]
Neurochem Res. 2017 Aug;42(8):2305-2313.
Glycogen synthase kinase 3beta (GSK-3beta) is a key downstream protein in the PI3K/Akt pathway. Phosphorylation of serine 9 of GSK-3beta (GSK-3beta activity inhibition) promotes cell survival. In this study, we examined changes in expressions of GSK-3beta and phosphorylation of GSK-3beta (p-GSK-3beta) in the gerbil hippocampal CA1 area after 5 min of transient cerebral ischemia. GSK-3beta immunoreactivity in the CA1 area was increased in pyramidal cells at 6 h after ischemia-reperfusion. It was decreased in CA1 pyramidal cells from 12 h after ischemia-reperfusion, and hardly detected in the CA1 pyramidal cells at 5 days after ischemia-reperfusion. p-GSK-3beta immunoreactivity was slightly decreased in CA1 pyramidal cells at 6 and 12 h after ischemia-reperfusion. It was significantly increased in these cells at 1 and 2 days after ischemia-reperfusion. Five days after ischemia-reperfusion, p-GSK-3beta immunoreactivity was hardly found in CA1 pyramidal cells. However, p-GSK-3beta immunoreactivity was strongly expressed in astrocytes primarily distributed in strata oriens and radiatum. In conclusion, GSK-3beta and p-GSK-3beta were significantly changed in pyramidal cells and/or astrocytes in the gerbil hippocampal CA1 area following 5 min of transient cerebral ischemia. This finding indicates that GSK-3beta and p-GSK-3beta are closely related to delayed neuronal death.
GSK-3-mediated phosphorylation couples ER-Golgi transport and nuclear stabilization of the CREB-H transcription factor to mediate apolipoprotein secretion.[Pubmed:28381424]
Mol Biol Cell. 2017 Jun 1;28(11):1565-1579.
CREB-H, an ER-anchored transcription factor, plays a key role in regulating secretion in metabolic pathways, particularly triglyceride homeostasis. It controls the production both of secretory pathway components and cargoes, including apolipoproteins ApoA-IV and ApoC-II, contributing to VLDL/HDL distribution and lipolysis. The key mechanism controlling CREB-H activity involves its ER retention and forward transport to the Golgi, where it is cleaved by Golgi-resident proteases, releasing the N-terminal product, which traffics to the nucleus to effect transcriptional responses. Here we show that a serine-rich motif termed the P-motif, located in the N-terminus between serines 73 and 90, controls release of the precursor transmembrane form from the ER and its forward transport to the Golgi. This motif is subject to GSK-3 phosphorylation, promoting ER retention, while mutation of target serines and drug inhibition of GSK-3 activity coordinately induce both forward transport of the precursor and cleavage, resulting in nuclear import. We previously showed that for the nuclear product, the P-motif is subject to multiple phosphorylations, which regulate stability by targeting the protein to the SCF(Fbw1a) E3 ubiquitin ligase. Thus phosphorylation at the P-motif provides integrated control of CREB-H function, coupling intercompartmental transport in the cytoplasm with stabilization of the active form in the nucleus.
SLM, a novel carbazole-based fluorophore attenuates okadaic acid-induced tau hyperphosphorylation via down-regulating GSK-3beta activity in SH-SY5Y cells.[Pubmed:28359686]
Eur J Pharm Sci. 2017 Dec 15;110:101-108.
Phosphorylated tau dissociates from microtubules and aggregates to form neurofibrillary tangles resulting in neuronal toxicity and cognitive deficits. Attenuating tau hyperphosphorylation is considered as an effective therapeutic approach for Alzheimer's disease (AD). From our previous study, SLM, a carbazole-based fluorophore prevents Abeta aggregation, reduced glycogen synthase kinase-3beta (GSK-3beta) activity and tau hyperphosphorylation in triple transgenic mouse model of AD. However, the mechanism by which SLM attenuates tau hyperphosphorylation warrants further investigation. In the current study, we intend to evaluate the effects of SLM against okadaic acid (OA)-induced tau hyperphosphorylation and microtubules instability in human neuroblastoma (SH-SY5Y) cells. The results showed that, SLM reduced the OA-induced cell neurotoxicity and tau hyperphosphorylation in SH-SY5Y cells. SLM treatment down-regulated GSK-3beta activity. However, in the presence of GSK-3beta inhibitor (SB216763, 10muM), SLM treatment could not reduce GSK-3beta activity and tau hyperphosphorylation as compared with SB216763 treatment alone. Furthermore, SLM treatment also ameliorated OA-induced microtubules instability and cytoskeleton damage. Collectively, SLM attenuated OA-induced tau hyperphosphorylation via down-regulating GSK-3beta activity in SH-SY5Y cells. Therefore, this study supports SLM as a potential compound for AD and other tau pathology-related neurodegenerative disorders.
Identification and characterization of a selective peroxisome proliferator-activated receptor beta/delta (NR1C2) antagonist.[Pubmed:17975020]
Mol Endocrinol. 2008 Feb;22(2):523-9.
The identification of small molecule ligands for the peroxisome proliferator-activated receptors (PPARs) has been instrumental in elucidating their biological roles. In particular, agonists have been the focus of much of the research in the field with relatively few antagonists being described and all of those being selective for PPARalpha or PPARgamma. The comparison of these agonist and antagonist ligands in cellular and animal systems has often led to surprising results and new insights into the biology of the PPARs. The PPARbeta/delta receptor is emerging as an important regulator of energy metabolism, inflammation, and cell growth and differentiation; however, only agonist ligands have been described for this receptor thus far. Here we describe the first report of a PPARbeta/delta small molecule antagonist ligand. This antagonist ligand will be a useful tool for elucidating the biological roles of PPARbeta/delta.
PPAR-delta in Vascular Pathophysiology.[Pubmed:19132133]
PPAR Res. 2008;2008:164163.
Peroxisome proliferator-activated receptors belong to the superfamily of ligand-dependent nuclear receptor transcription factors, which include three subtypes: PPAR-alpha, beta/delta, and gamma. PPAR-delta, play important roles in the regulation of cell growth and differentiation as well as tissue wound and repair. Emerging evidence has also demonstrated that PPAR-delta is implicated in lipids and glucose metabolism. Most recently, the direct effects of PPAR-delta on cardiovascular processes such as endothelial function and angiogenesis have also been investigated. Therefore, it is suggested that PPAR-delta may have critical roles in cardiovascular pathophysiology and is a potential target for therapeutic intervention of cardiovascular disorders such as atherosclerosis.


