Taxinine MCAS# 135730-55-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 135730-55-1 | SDF | Download SDF |
| PubChem ID | 131908 | Appearance | Powder |
| Formula | C35H42O14 | M.Wt | 686.70 |
| Type of Compound | Diterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
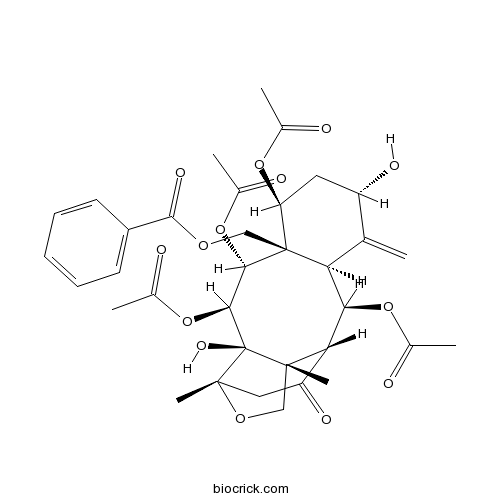
| SMILES | CC(=O)OC1CC(C(=C)C2C1(C(C(C3(C4(CC(=O)C(C2OC(=O)C)C3(CO4)C)C)O)OC(=O)C)OC(=O)C)COC(=O)C5=CC=CC=C5)O | ||
| Standard InChIKey | NLPSSNXRGVDJMP-NTOIUUNRSA-N | ||
| Standard InChI | InChI=1S/C35H42O14/c1-17-23(40)13-25(46-18(2)36)34(16-44-31(42)22-11-9-8-10-12-22)26(17)28(47-19(3)37)27-24(41)14-33(7)35(43,32(27,6)15-45-33)30(49-21(5)39)29(34)48-20(4)38/h8-12,23,25-30,40,43H,1,13-16H2,2-7H3/t23-,25-,26-,27-,28+,29-,30-,32-,33-,34+,35-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Taxinine M is a natural product from Chinese yew Taxus chinensis. |
| In vitro | Simultaneous determination of seven taxoids in rat plasma by UPLC-MS/MS and pharmacokinetic study after oral administration of Taxus yunnanensis extracts.[Pubmed: 25645339]J Pharm Biomed Anal. 2015 Mar 25;107:346-54.
|
| Structure Identification | Phytochemistry. 1999 Dec;52(8):1565-9.Taxane diterpenoids from the seeds of Chinese yew Taxus chinensis.[Pubmed: 10647221]The taxoid chinentaxunine has been isolated from the seeds of Chinese yew Taxus chinensis, and its structure determined on the basis of spectral and chemical methods. In addition, the known taxol C, paclitaxel, 10-deacetyl taxol A, 10-deacetyl-7-epitaxol, 10-deacetyl-10-oxo-7-epi-taxol, Taxinine M, taxchinin A, 10-deacetyl taxinine B and taxuspine X were also isolated and identified from this source. |

Taxinine M Dilution Calculator

Taxinine M Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.4562 mL | 7.2812 mL | 14.5624 mL | 29.1248 mL | 36.406 mL |
| 5 mM | 0.2912 mL | 1.4562 mL | 2.9125 mL | 5.825 mL | 7.2812 mL |
| 10 mM | 0.1456 mL | 0.7281 mL | 1.4562 mL | 2.9125 mL | 3.6406 mL |
| 50 mM | 0.0291 mL | 0.1456 mL | 0.2912 mL | 0.5825 mL | 0.7281 mL |
| 100 mM | 0.0146 mL | 0.0728 mL | 0.1456 mL | 0.2912 mL | 0.3641 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Palonosetron hydrochloride
Catalog No.:BCN2171
CAS No.:135729-62-3
- Palonosetron
Catalog No.:BCC1834
CAS No.:135729-61-2
- MDL 29,913
Catalog No.:BCC5729
CAS No.:135721-56-1
- ML 228
Catalog No.:BCC2435
CAS No.:1357171-62-0
- c-Met inhibitor 1
Catalog No.:BCC1488
CAS No.:1357072-61-7
- N-Acetylglycyl-D-glutamic acid
Catalog No.:BCC6634
CAS No.:135701-69-8
- HG-14-10-04
Catalog No.:BCC5392
CAS No.:1356962-34-9
- AZD-3463
Catalog No.:BCC3907
CAS No.:1356962-20-3
- ent-3-Oxokaurane-16,17-diol
Catalog No.:BCN6188
CAS No.:135683-73-7
- Narchinol B
Catalog No.:BCN7796
CAS No.:1356822-09-7
- Fmoc-Cha-OH
Catalog No.:BCC3160
CAS No.:135673-97-1
- 17-Methylparsonsianidine
Catalog No.:BCN2093
CAS No.:135637-68-2
- OG-L002
Catalog No.:BCC4549
CAS No.:1357302-64-7
- Nonin A
Catalog No.:BCN7149
CAS No.:1357351-29-1
- RGFP966
Catalog No.:BCC3991
CAS No.:1357389-11-7
- Boc-Glu(OBzl)-OH
Catalog No.:BCC3389
CAS No.:13574-13-5
- Isoforsythiaside
Catalog No.:BCN5413
CAS No.:1357910-26-9
- Koumine
Catalog No.:BCN6190
CAS No.:1358-76-5
- erythro-Guaiacylglycerol beta-dihydroconiferyl ether
Catalog No.:BCN7025
CAS No.:135820-77-8
- Blumenol C glucoside
Catalog No.:BCN6189
CAS No.:135820-80-3
- H-D-Asp(OtBu)-OtBu.HCl
Catalog No.:BCC2898
CAS No.:135904-71-1
- Lupinol C
Catalog No.:BCN4809
CAS No.:135905-53-2
- RP 67580
Catalog No.:BCC7134
CAS No.:135911-02-3
- SR 2211
Catalog No.:BCC6310
CAS No.:1359164-11-6
Taxumairol M: a new bicyclic taxoid from seeds of Taxus mairei.[Pubmed:10483381]
Planta Med. 1999 Aug;65(6):582-4.
In addition to taxol A (1), 10-deacetyltaxol A (2), 10-deacetyl-7-epi-taxol A (3), 10-deacetylbaccatin III (5), taxuspine Z, taxezopidine G, 5 alpha-cinnamoyloxy-9 alpha,10 beta,13 alpha-triacetoxytaxa-4(20)11-diene, taxinine J, and Taxinine M, a new bicyclic taxoid named taxumairol M (4), has been isolated from the seeds of Taxus mairei. The structure of 4 was determined on the basis of spectral analysis.
Taxane diterpenoids from the seeds of Chinese yew Taxus chinensis.[Pubmed:10647221]
Phytochemistry. 1999 Dec;52(8):1565-9.
The taxoid chinentaxunine has been isolated from the seeds of Chinese yew Taxus chinensis, and its structure determined on the basis of spectral and chemical methods. In addition, the known taxol C, paclitaxel, 10-deacetyl taxol A, 10-deacetyl-7-epitaxol, 10-deacetyl-10-oxo-7-epi-taxol, Taxinine M, taxchinin A, 10-deacetyl taxinine B and taxuspine X were also isolated and identified from this source.
Simultaneous determination of seven taxoids in rat plasma by UPLC-MS/MS and pharmacokinetic study after oral administration of Taxus yunnanensis extracts.[Pubmed:25645339]
J Pharm Biomed Anal. 2015 Mar 25;107:346-54.
A rapid, sensitive and reliable method has been developed and validated for the simultaneous determination of seven taxoids including 10-deacetylbaccatin III (10-DAB III), baccatin III, 5-epi-canadensene, Taxinine M, 10-deacetyltaxol (10-DAT), cephalomannine and paclitaxel in rat plasma using docetaxel as the internal standard (IS). The plasma samples were pretreated by liquid-liquid extraction with methyl tert-butyl ether. The chromatographic separation was achieved on a C18 column (50 mm x 2.1 mm, 1.8 mum, Waters, USA) with a gradient elution program consisting of methanol and water (containing 0.1% formic acid) at a flow rate of 0.2 mL/min. Detection was performed under the selected reaction monitoring (SRM) scan using an electrospray ionization (ESI) in the positive ion mode. The mass transitions were as follows: m/z 567.4-->444.9 for 10-DAB III, m/z 609.0-->549.3 for baccatin III, m/z 617.4-->496.9 for 5-epi-canadensene, m/z 709.6-->649.3 for Taxinine M, m/z 834.8-->307.9 for 10-DAT, m/z 854.5-->285.4 for cephalomannine, m/z 876.8-->307.3 for paclitaxel and m/z 830.8-->549.6 for IS, respectively. All calibration curves exhibited good linearity (r(2)>0.99) over a wide concentration range for all components. The intra-day and inter-day precisions at three different levels were both less than 14.3% in terms of relative standard deviation (RSD) and the accuracies ranged from -8.3% to 14.8% in terms of relative error (RE). The extraction recoveries of the seven compounds ranged from 62.5% to 100.5%. The developed method was successfully applied to the pharmacokinetic study of the seven taxoids in rat plasma after oral administration of the crude extract of the twigs and leaves of Taxus yunnanensis.
Taxines from the needles of Taxus wallichiana.[Pubmed:11738401]
Phytochemistry. 2001 Dec;58(8):1167-70.
A taxine, 5 alpha O-(3'-dimethylamino-3'-phenylpropionyl) Taxinine M (1) together with two known compounds 7-O-acetyltaxine A (2) and 2 alpha-acetoxy-2' beta-deacetylaustrospicatine (3) were isolated from the needles of the Himalayan yew, Taxus wallichiana Zucc. Their structures were elucidated on the basis of the NMR spectral data, ESI-MS/MS analysis and chemical methods. Compounds 1 and 3 showed moderate cytotoxic activity against the lung cancer cell line A549 in vitro.
Minor taxoids from Taxus wallichiana.[Pubmed:7673940]
J Nat Prod. 1995 Jun;58(6):934-9.
The needles of Taxus wallichiana afforded a new analogue of Taxinine M [1a] and two derivatives of brevifoliol [2a, 3a]. The conformation of 3a was investigated by nmr spectroscopy with the aid of variable-temperature experiments and in situ reactions.
Multidrug resistance reversal activity of taxoids from Taxus cuspidata in KB-C2 and 2780AD cells.[Pubmed:10874217]
Jpn J Cancer Res. 2000 Jun;91(6):638-42.
Some non-taxol-type taxoids having neither an oxetane ring at C-4 and C-5 nor an N-acylphenyl-isoserine group at C-13, such as taxuspine C, 2'-desacetoxyaustrospicatine, and 2-desacetoxytaxinine J, which were isolated from the Japanese yew Taxus cuspidata, increased cellular accu-mulation of vincristine (VCR) in multidrug-resistant 2780AD cells as potently as verapamil, and efficiently inhibited [(3)H]azidopine photolabeling of P-glycoprotein (P-gp). Taxuspine C, 2'-desacetoxyaustrospicatine, and 2-desacetoxytaxinine J at 10 microM completely reversed the resistance to colchicine, VCR, and taxol in KB-C2 cells, which overexpress P-gp, while taxinine and Taxinine M showed no effect. Taxuspine C, 2'-desacetoxyaustrospicatine, and 2-desacetoxytaxinine J may be candidate pharmaceuticals for reversing multidrug resistance (MDR) and also may be good modifiers of MDR in cancer chemotherapy.


