Nonin ACAS# 1357351-29-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1357351-29-1 | SDF | Download SDF |
| PubChem ID | 129317369 | Appearance | Powder |
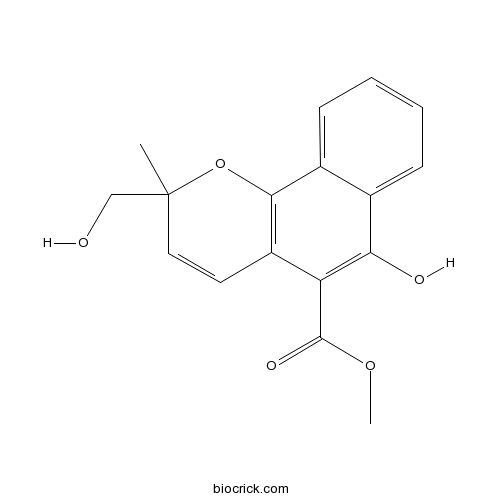
| Formula | C17H16O5 | M.Wt | 300.31 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | methyl 6-hydroxy-2-(hydroxymethyl)-2-methylbenzo[h]chromene-5-carboxylate | ||
| SMILES | CC1(C=CC2=C(O1)C3=CC=CC=C3C(=C2C(=O)OC)O)CO | ||
| Standard InChIKey | IDZKIDTWFRCHKW-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C17H16O5/c1-17(9-18)8-7-12-13(16(20)21-2)14(19)10-5-3-4-6-11(10)15(12)22-17/h3-8,18-19H,9H2,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Nonin A Dilution Calculator

Nonin A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.3299 mL | 16.6495 mL | 33.2989 mL | 66.5978 mL | 83.2473 mL |
| 5 mM | 0.666 mL | 3.3299 mL | 6.6598 mL | 13.3196 mL | 16.6495 mL |
| 10 mM | 0.333 mL | 1.6649 mL | 3.3299 mL | 6.6598 mL | 8.3247 mL |
| 50 mM | 0.0666 mL | 0.333 mL | 0.666 mL | 1.332 mL | 1.6649 mL |
| 100 mM | 0.0333 mL | 0.1665 mL | 0.333 mL | 0.666 mL | 0.8325 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- OG-L002
Catalog No.:BCC4549
CAS No.:1357302-64-7
- Taxinine M
Catalog No.:BCN6942
CAS No.:135730-55-1
- Palonosetron hydrochloride
Catalog No.:BCN2171
CAS No.:135729-62-3
- Palonosetron
Catalog No.:BCC1834
CAS No.:135729-61-2
- MDL 29,913
Catalog No.:BCC5729
CAS No.:135721-56-1
- ML 228
Catalog No.:BCC2435
CAS No.:1357171-62-0
- c-Met inhibitor 1
Catalog No.:BCC1488
CAS No.:1357072-61-7
- N-Acetylglycyl-D-glutamic acid
Catalog No.:BCC6634
CAS No.:135701-69-8
- HG-14-10-04
Catalog No.:BCC5392
CAS No.:1356962-34-9
- AZD-3463
Catalog No.:BCC3907
CAS No.:1356962-20-3
- ent-3-Oxokaurane-16,17-diol
Catalog No.:BCN6188
CAS No.:135683-73-7
- Narchinol B
Catalog No.:BCN7796
CAS No.:1356822-09-7
- RGFP966
Catalog No.:BCC3991
CAS No.:1357389-11-7
- Boc-Glu(OBzl)-OH
Catalog No.:BCC3389
CAS No.:13574-13-5
- Isoforsythiaside
Catalog No.:BCN5413
CAS No.:1357910-26-9
- Koumine
Catalog No.:BCN6190
CAS No.:1358-76-5
- erythro-Guaiacylglycerol beta-dihydroconiferyl ether
Catalog No.:BCN7025
CAS No.:135820-77-8
- Blumenol C glucoside
Catalog No.:BCN6189
CAS No.:135820-80-3
- H-D-Asp(OtBu)-OtBu.HCl
Catalog No.:BCC2898
CAS No.:135904-71-1
- Lupinol C
Catalog No.:BCN4809
CAS No.:135905-53-2
- RP 67580
Catalog No.:BCC7134
CAS No.:135911-02-3
- SR 2211
Catalog No.:BCC6310
CAS No.:1359164-11-6
- Caulophylline B
Catalog No.:BCN7499
CAS No.:1359978-55-4
- Phenazopyridine HCl
Catalog No.:BCC4698
CAS No.:136-40-3
Clinical Validation of Heart Rate Apps: Mixed-Methods Evaluation Study.[Pubmed:28842392]
JMIR Mhealth Uhealth. 2017 Aug 25;5(8):e129.
BACKGROUND: Photoplethysmography (PPG) is a proven way to measure heart rate (HR). This technology is already available in smartphones, which allows measuring HR only by using the smartphone. Given the widespread availability of smartphones, this creates a scalable way to enable mobile HR monitoring. An essential precondition is that these technologies are as reliable and accurate as the current clinical (gold) standards. At this moment, there is no consensus on a gold standard method for the validation of HR apps. This results in different validation processes that do not always reflect the veracious outcome of comparison. OBJECTIVE: The aim of this paper was to investigate and describe the necessary elements in validating and comparing HR apps versus standard technology. METHODS: The FibriCheck (Qompium) app was used in two separate prospective nonrandomized studies. In the first study, the HR of the FibriCheck app was consecutively compared with 2 different Food and Drug Administration (FDA)-cleared HR devices: the Nonin oximeter and the AliveCor Mobile ECG. In the second study, a next step in validation was performed by comparing the beat-to-beat intervals of the FibriCheck app to a synchronized ECG recording. RESULTS: In the first study, the HR (BPM, beats per minute) of 88 random subjects consecutively measured with the 3 devices showed a correlation coefficient of .834 between FibriCheck and Nonin, .88 between FibriCheck and AliveCor, and .897 between Nonin And AliveCor. A single way analysis of variance (ANOVA; P=.61 was executed to test the hypothesis that there were no significant differences between the HRs as measured by the 3 devices. In the second study, 20,298 (ms) R-R intervals (RRI)-peak-to-peak intervals (PPI) from 229 subjects were analyzed. This resulted in a positive correlation (rs=.993, root mean square deviation [RMSE]=23.04 ms, and normalized root mean square error [NRMSE]=0.012) between the PPI from FibriCheck and the RRI from the wearable ECG. There was no significant difference (P=.92) between these intervals. CONCLUSIONS: Our findings suggest that the most suitable method for the validation of an HR app is a simultaneous measurement of the HR by the smartphone app and an ECG system, compared on the basis of beat-to-beat analysis. This approach could lead to more correct assessments of the accuracy of HR apps.
Comparative Evaluation of Accuracy of Pulse Oximeters and Factors Affecting Their Performance in a Tertiary Intensive Care Unit.[Pubmed:28764215]
J Clin Diagn Res. 2017 Jun;11(6):OC05-OC08.
INTRODUCTION: Pulse oximetry is a widely used tool, unfortunately there is a paucity of data investigating its accuracy in Intensive Care Units (ICU) and if they are able to meet mandated FDA criteria as claimed by them in critically ill patients. AIM: To assess bias, precision and accuracy of pulse oximeters used in ICU and factors affecting them. MATERIALS AND METHODS: A prospective cohort study, including 129 patients admitted to the ICU of a tertiary referral centre. Pulse oximetry and blood gas were done simultaneously. Pulse oximetry was done using two pulse oximetres: Nonin And Philips. All physiological variables like haemoglobin, lactate, use of vasopressors and blood pressure were recorded. Bland Altman curves were constructed to determine bias and limits of agreement. Effect of physiological variables on bias and difference between performance characteristics of bias was determined using SPSS. RESULTS: Pulse oximetry overestimated arterial oxygen saturation (SaO2) by 1.44%. There was negative correlation between bias and SaO2 (r=-0.32) and positive correlation with lactate (r=0.16). The Philips pulse oximeter had significant higher bias and variability than Nonin pulse oximeter. (2.49+/-2.99 versus 0.46+/-1.68, mean difference = 1.98, 95% C.I. = 1.53 - 2.43, p-value <0.001). CONCLUSION: Pulse oximetry overestimates SaO2. Bias tends to increase with rising lactate and hypoxia. There is heterogeneity in performance of various pulse oximetry devices in ICU.


