TetrahydroauroglaucinCAS# 40434-07-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 40434-07-9 | SDF | Download SDF |
| PubChem ID | 14355117 | Appearance | Yellow powder |
| Formula | C19H26O3 | M.Wt | 302.4 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
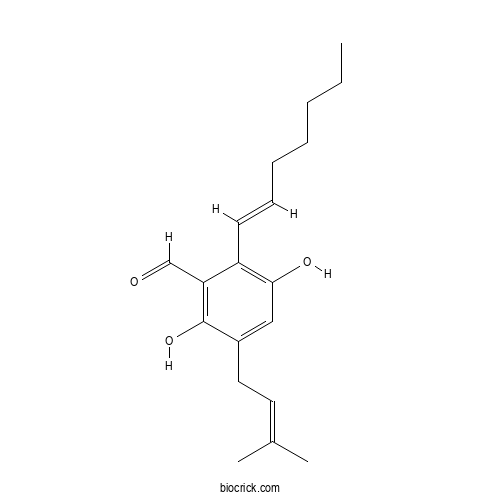
| Chemical Name | 2-[(E)-hept-1-enyl]-3,6-dihydroxy-5-(3-methylbut-2-enyl)benzaldehyde | ||
| SMILES | CCCCCC=CC1=C(C=C(C(=C1C=O)O)CC=C(C)C)O | ||
| Standard InChIKey | FYGPFTSGVSZKAJ-CMDGGOBGSA-N | ||
| Standard InChI | InChI=1S/C19H26O3/c1-4-5-6-7-8-9-16-17(13-20)19(22)15(12-18(16)21)11-10-14(2)3/h8-10,12-13,21-22H,4-7,11H2,1-3H3/b9-8+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Tetrahydroauroglaucin Dilution Calculator

Tetrahydroauroglaucin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.3069 mL | 16.5344 mL | 33.0688 mL | 66.1376 mL | 82.672 mL |
| 5 mM | 0.6614 mL | 3.3069 mL | 6.6138 mL | 13.2275 mL | 16.5344 mL |
| 10 mM | 0.3307 mL | 1.6534 mL | 3.3069 mL | 6.6138 mL | 8.2672 mL |
| 50 mM | 0.0661 mL | 0.3307 mL | 0.6614 mL | 1.3228 mL | 1.6534 mL |
| 100 mM | 0.0331 mL | 0.1653 mL | 0.3307 mL | 0.6614 mL | 0.8267 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 5,4'-Dihydroxy-6,7,8,3'-tetramethoxyflavone
Catalog No.:BCX0516
CAS No.:16520-78-8
- 5,7-Dihydroxy-3,8,3',4'-tetramethoxyflavone
Catalog No.:BCX0515
CAS No.:42923-42-2
- Xanthomicrol
Catalog No.:BCX0514
CAS No.:16545-23-6
- N-Acetyl-D-mannosamine
Catalog No.:BCX0513
CAS No.:7772-94-3
- N-acetyl-D-galactosamine
Catalog No.:BCX0512
CAS No.:1811-31-0
- D-Galactosamine hydrochloride
Catalog No.:BCX0511
CAS No.:1772-03-8
- D-Mannosamine hydrochloride
Catalog No.:BCX0510
CAS No.:5505-63-5
- Moracin N
Catalog No.:BCX0509
CAS No.:135248-05-4
- (S)-5-Hydroxy-1-(4-hydroxy-3-methoxyphenyl)-7-phenylheptan-3-one
Catalog No.:BCX0508
CAS No.:1220110-76-8
- 1β-Hydroxy-8α-methoxyeremophila-7(11),9-dien-12,8β-olide
Catalog No.:BCX0507
CAS No.:849700-45-4
- Methylconiferin
Catalog No.:BCX0506
CAS No.:883150-46-7
- Iristectorin B
Catalog No.:BCX0505
CAS No.:94396-09-5
- Sanggenon F
Catalog No.:BCX0518
CAS No.:85889-03-8
- 4-Hydroxyphenylpyruvic acid
Catalog No.:BCX0519
CAS No.:156-39-8
- Isodihydroauroglaucin
Catalog No.:BCX0520
CAS No.:74886-31-0
- Demethoxysudachitin
Catalog No.:BCX0521
CAS No.:4323-80-2
- Oxyphyllenodiol B
Catalog No.:BCX0522
CAS No.:363610-32-6
- Labda-12E,14-dien-16,15-olid-17-oic acid
Catalog No.:BCX0523
CAS No.:1855905-16-6
- Butyl rosmarinate
Catalog No.:BCX0524
CAS No.:222713-83-9
- Dihydroauroglaucin
Catalog No.:BCX0525
CAS No.:77102-91-1
- (E)-5-Hydroxy-6-isoprenyl-2-(pent-1-en-1-yl)benzofuran-4-carbaldehyde
Catalog No.:BCX0526
CAS No.:916602-30-7
- 5-(1-Hydroxypropan-2-yl)-2-methylphenol
Catalog No.:BCX0527
CAS No.:111044-80-5
- 5,7,4'-Trihydroxy-3,8-dimethoxyflavone
Catalog No.:BCX0528
CAS No.:14965-09-4
- 4-Hydroxythonningianin B
Catalog No.:BCX0529
CAS No.:2329726-95-4
The marine-derived compound TAG alleviates Parkinson's disease by restoring RUBCN-mediated lipid metabolism homeostasis.[Pubmed:38538717]
Acta Pharmacol Sin. 2024 Mar 27.
Parkinson's disease (PD) is the second most common neurodegenerative disease, and its prevalence is increasing. Currently, no effective therapies for PD exist. Marine-derived natural compounds are considered important resources for the discovery of new drugs due to their distinctive structures and diverse activities. In this study, Tetrahydroauroglaucin (TAG), a polyketide isolated from a marine sponge, was found to have notable neuroprotective effects on MPTP/MPP(+)-induced neurotoxicity. RNA sequencing analysis and metabolomics revealed that TAG significantly improved lipid metabolism disorder in PD models. Further investigation indicated that TAG markedly decreased the accumulation of lipid droplets (LDs), downregulated the expression of RUBCN, and promoted autophagic flux. Moreover, conditional knockdown of Rubcn notably attenuated PD-like symptoms and the accumulation of LDs, accompanied by blockade of the neuroprotective effect of TAG. Collectively, our results first indicated that TAG, a promising PD therapeutic candidate, could suppress the accumulation of LDs through the RUBCN-autophagy pathway, which highlighted a novel and effective strategy for PD treatment.
Characterization of Hypolipidemic Phenol Analogues from Fermented Tea by Eurotium cristatum.[Pubmed:36613264]
Foods. 2022 Dec 22;12(1):49.
Fuzhuan brick tea (FBT), a type of black tea, is a traditional beverage in China, especially popular among frontier ethnic groups. FBT is well-known for its health benefits, such as hypoglycemic, anti-hypertensive, anti-inflammatory, diuretic, and detoxification effects. Nevertheless, the underlying mechanisms on the molecular level are still elusive and the key compounds responsible for the health benefits are unidentified. Previous studies have mainly focused on functional studies of the water extract. However, FBT is typically cooked with butter or milk. Therefore, we hypothesized that some lipophilic components in FBT, which can be absorbed through the co-consumption of butter or milk, may play an important role in the health benefits. The present study aimed to investigate whether the liposoluble extract of FBT alleviates symptoms related to metabolic diseases and to identify the active compounds involved. By comparing the high-performance liquid chromatography (HPLC) profiles of water, milk and hexane extract, some low polarity peaks were observed in the milk and hexane extracts. Furthermore, the hexane extract treatment alleviated body weight gain, serum total cholesterol and triglyceride levels, and inhibited the accumulation of hepatic fat granules in a high-fat diet (HFD)-induced C57BL/6N mouse model. In order to identify the key functional lipophilic compounds in FBT, the hexane extract of FBT was subjected to chemical characterization. Four phenol analogs were characterized, namely, isodihydroauroglaucin (1), dihydroauroglaucin (2), Tetrahydroauroglaucin (3), and flavoglaucin (4). Compounds 1 and 4 reduced the levels of total cholesterol and triglyceride in vivo. Both compounds also inhibited the high-fat diet-induced body weight gain and accumulation of fat granules in the liver of C57BL/6N mice. Isodihydroauroglaucin and flavoglaucin have therefore been identified as bioactive ingredients that contribute to the health benefits of FBT.
Dihydroauroglaucin Isolated from the Mediterranean Sponge Grantia compressa Endophyte Marine Fungus Eurotium chevalieri Inhibits Migration of Human Neuroblastoma Cells.[Pubmed:35335990]
Pharmaceutics. 2022 Mar 11;14(3):616.
Cancer cell migration is a hallmark of the aggressiveness and progression of malignancies such as high-risk neuroblastoma. Given the lack of effective therapeutic solutions to counteract cancer progression, basic research aims to identify novel bioactive molecules with inhibitory potential on cancer cell migration. In this context, this work investigated the role of members of the salicylaldehyde secondary metabolite set from the sponge endophyte fungus Eurotium chevalieri MUT 2316 as potential inhibitors of human neuroblastoma SH-SY5Y cell migration. Since Tetrahydroauroglaucin (TAG) and dihydroauroglaucin (DAG) were isolated in large amounts, both were evaluated for their anticancer properties towards SH-SY5Y cells. Both molecules were found to be non-cytotoxic by MTT assay and cytofluorimetric analysis. Moreover, DAG showed efficacy in inhibiting the highly migratory phenotype of SH-SY5Y cells by wound healing assay; whereas TAG, although structurally similar to DAG, showed no anti-migratory effect. Therefore, this work provides good reasons to conduct further in vitro and in vivo studies focusing on DAG as a potentially useful migrastatic natural marine molecule.
Bioactive Metabolites From Acid-Tolerant Fungi in a Thai Mangrove Sediment.[Pubmed:33552019]
Front Microbiol. 2021 Jan 22;11:609952.
Despite being potentially useful extremophile resources, there have been few reports on acid-tolerant fungi and their bioactive metabolites. Acidophilic/aciduric fungi (n = 237) were isolated from Thai mangrove sediments in an acidic medium. Using fungal identification technology (including morphologic observation, chemical screening, and sequence comparisons) all the isolates were identified and 41 representative isolates were selected for analysis of the phylogenetic relationships (ITS rDNA, beta-tubulin, calmodulin, and actin gene sequences). There were seven genera identified - Penicillium; Aspergillus; Talaromyces; Cladosporium; Allophoma; Alternaria; and Trichoderma - in four taxonomic orders of the phylum Ascomycota, and Penicillium, Aspergillus, and Talaromyces were the dominant genera. Acidity tolerance was evaluated and 95% of the isolates could grow under extremely acidic conditions (pH 2). Six strains were classed as acidophilic fungi that cannot survive under pH 7, all of which had an extraordinarily close genetic relationship and belonged to the genus Talaromyces. This is the first report on the acidophilic characteristics of this genus. The antimicrobial, anti-tumor, and antiviral activities of the fermentation extracts were evaluated. Nearly three-quarters of the extracts showed cytotoxic activity, while less than a quarter showed antimicrobial or anti-H1N1 activity. The typical aciduric fungus Penicillium oxalicum OUCMDZ-5207 showed similar growth but completely different chemical diversity at pH 3 and 7. The metabolites of OUCMDZ-5207 that were obtained only at pH 3 were identified as Tetrahydroauroglaucin (1), flavoglaucin (2), and auroglaucin (3), among which auroglaucin showed strong selective inhibition of A549 cells with an IC(50) value of 5.67 muM. These results suggest that acid stress can activate silent gene clusters to expand the diversity of secondary metabolites, and the bioprospecting of aciduric/acidophilic microorganism resources in Thai mangrove sediments may lead to the discovery of compounds with potential medicinal applications.
Marine Fungus Aspergillus chevalieri TM2-S6 Extract Protects Skin Fibroblasts from Oxidative Stress.[Pubmed:32911774]
Mar Drugs. 2020 Sep 8;18(9):460.
The strain Aspergillus chevalieri TM2-S6 was isolated from the sponge Axinella and identified according to internal transcribed spacer (ITS) molecular sequence homology with Aspergillus species from the section Restricti. The strain was cultivated 9 days on potato dextrose broth (PDB), and the medium evaluated as antioxidant on primary normal human dermal fibroblasts (NHDF). The cultivation broth was submitted to sterile filtration, lyophilized and used without any further processing to give the Aspergillus chevalieri TM2-S6 cultivation broth ingredient named ACBB. ACCB contains two main compounds: Tetrahydroauroglaucin and flavoglaucin. Under oxidative stress, ACCB showed a significant promotion of cell viability. To elucidate the mechanism of action, the impact on a panel of hundreds of genes involved in fibroblast physiology was evaluated. Thus, ACCB stimulates cell proliferation (VEGFA, TGFB3), antioxidant response (GPX1, SOD1, NRF2), and extracellular matrix organization (COL1A1, COL3A1, CD44, MMP14). ACCD also reduced aging (SIRT1, SIRT2, FOXO3). These findings indicate that Aspergillus chevalieri TM2-S6 cultivation broth exhibits significant in vitro skin protection of human fibroblasts under oxidative stress, making it a potential cosmetic ingredient.
Bio-Guided Fractionation of Prenylated Benzaldehyde Derivatives as Potent Antimicrobial and Antibiofilm from Ammi majus L. Fruits-Associated Aspergillus amstelodami.[Pubmed:31739552]
Molecules. 2019 Nov 14;24(22):4118.
Ammi majus L.; Family Apiaceae; is a plant indigenous to Egypt. Its fruits contain bioactive compounds such as furanocoumarins and flavonoids of important biological activities. An endophytic fungus was isolated from the fruits and identified as Aspergillus amstelodami (MK215708) by morphology, microscopical characterization, and molecular identification. To our knowledge this is the first time an endophytic fungus has been isolated from the fruits. The antimicrobial activity of the Ammi majus ethanol fruits extract (AME) and fungal ethyl acetate extract (FEA) were investigated, where the FEA showed higher antimicrobial activity, against all the tested standard strains. Phytochemical investigation of the FEA extract yielded five prenylated benzaldehyde derivative compounds isolated for the first time from this species: Dihydroauroglaucin (1), Tetrahydroauroglaucin (2), 2-(3,6-dihydroxyhepta-1,4-dien-1-yl)-3,6-dihydroxy-5-(dimethylallyl)benzaldehyde (3), isoTetrahydroauroglaucin )4), and flavoglaucin (5). Structure elucidation was carried out using (1H- and 13C-NMR). Fractions and the major isolated compound 1 were evaluated for their antimicrobial and antibiofilm activity. Compound 1 showed high antimicrobial activity against Escherichia coli with minimum inhibitory concentration (MIC) = 1.95 microg/mL, Streptococcus mutans (MIC = 1.95 microg/mL), and Staphylococcus aureus (MIC = 3.9 microg/mL). It exhibited high antibiofilm activity with minimum biofilm inhibitory concentration (MBIC) = 7.81 microg/mL against Staphylococcus aureus and Escherichia coli biofilms and MBIC = 15.63 microg/mL against Streptococcus mutans and Candida albicans and moderate activity (MBIC = 31.25 microg/mL) against Pseudomonas aeruginosa biofilm. This reveals that dihydroauroglaucin, a prenylated benzaldehyde derivative, has a broad spectrum antimicrobial activity. In conclusion, it was observed that the MICs of the FEA are much lower than that of the AME against all susceptible strains, confirming that the antimicrobial activity of Ammi majus may be due to the ability of its endophytic fungi to produce effective secondary metabolites.
Two benzaldehyde derivatives and their artefacts from a gorgonian-derived Eurotium sp. fungus.[Pubmed:27627699]
Nat Prod Res. 2017 Feb;31(3):268-274.
Two new benzaldehyde derivatives, named 3'-OH-Tetrahydroauroglaucin (1) and(3'S*,4'R*)-6-(3',5-epoxy-4'-hydroxy-1'-heptenyl)-2-hydroxy-3-(3''-methyl-2''-butenyl)benzaldehyde (2), were isolated from a gorgonian-derived Eurotium sp. fungus. Their structures were determined by extensive spectroscopic analysis including NMR and MS spectra. Dissolved 1 in CDCl(3) for several days could be detected its 2H-chromene skeleton derivatives (1a/1b), a pair of enantiomers with opposite configurations at C-3'. Compound 2 was also found to chemically convert to a pair of epimers non-enzymatically. The plausible mechanism to form the 2H-chromene artefacts with racemisation at C-3' undergoing nucleophilic substitution (S(N)1) was proposed.
Benzyl derivatives with in vitro binding affinity for human opioid and cannabinoid receptors from the fungus Eurotium repens.[Pubmed:21667972]
J Nat Prod. 2011 Jul 22;74(7):1636-9.
Bioassay-guided fractionation of the fungus Eurotium repens resulted in the isolation of two new benzyl derivatives, (E)-2-(hept-1-enyl)-3-(hydroxymethyl)-5-(3-methylbut-2-enyl)benzene-1,4-diol (1) and (E)-4-(hept-1-enyl)-7-(3-methylbut-2-enyl)-2,3-dihydrobenzofuran-2,5-diol (2), along with seven known compounds (3-9) including five benzaldehyde compounds, flavoglaucin (3), Tetrahydroauroglaucin (4), dihydroauroglaucin (5), auroglaucin (6), and 2-(2',3-epoxy-1',3'- heptadienyl)-6-hydroxy-5-(3-methyl-2-butenyl)benzaldehyde (7), one diketopiperazine alkaloid, echinulin (8), and 5,7-dihydroxy-4-methylphthalide (9). The chemical structures of these compounds were established on the basis of extensive 1D and 2D NMR and HRMS data. Compounds 1-4 and 6 showed good binding affinity for human opioid or cannabinoid receptors. These findings have important implications for psychoactive studies with this class of compounds.
Antioxidants produced by Eurotium herbariorum of filamentous fungi used for the manufacture of karebushi, dried bonito (Katsuobushi).[Pubmed:19502740]
Biosci Biotechnol Biochem. 2009 Jun;73(6):1323-7.
Extracts prepared by culturing ten filamentous fungi from Aspergillus and Eurotium species isolated from dried bonito (katsuobushi) were examined for 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging capacity. The extracts prepared by culturing E. herbariorum NE-1 and NE-4, which are used in the molding process for the manufacture of karebushi (a kind of katsuobushi), were shown to have higher activity than the others. Five antioxidants were isolated from the extracts and identified as isodihydroauroglaucin (IDAG), auroglaucin (AG), dihydroauroglaucin (DAG), Tetrahydroauroglaucin (TAG), and flavoglaucin (FG) by (1)H-NMR, (13)C-NMR, and EI-MS analyses. Compared with alpha-tocopherol, the isolated antioxidants exhibited high antioxidative activity for the radical scavenging capacity of DPPH and superoxide, but low activity for inhibiting the autoxidation of docosahexaenoic acid (DHA). The isolated antioxidants were produced by the Eurotium species, but not by the Aspergillus species. DAG and TAG exhibited higher radical scavenging capacity than the other antioxidants and were abundantly contained in the extracts of E. herbariorum NE-1 and NE-4.


