Moracin NCAS# 135248-05-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 135248-05-4 | SDF | Download SDF |
| PubChem ID | 641376 | Appearance | Powder |
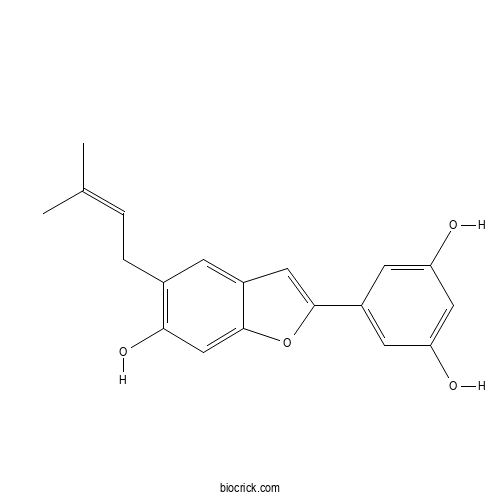
| Formula | C19H18O4 | M.Wt | 310.3 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 5-[6-hydroxy-5-(3-methylbut-2-enyl)-1-benzofuran-2-yl]benzene-1,3-diol | ||
| SMILES | CC(=CCC1=C(C=C2C(=C1)C=C(O2)C3=CC(=CC(=C3)O)O)O)C | ||
| Standard InChIKey | WBSCSIABHGPAMC-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C19H18O4/c1-11(2)3-4-12-5-13-8-18(23-19(13)10-17(12)22)14-6-15(20)9-16(21)7-14/h3,5-10,20-22H,4H2,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Moracin N Dilution Calculator

Moracin N Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.2227 mL | 16.1134 mL | 32.2269 mL | 64.4538 mL | 80.5672 mL |
| 5 mM | 0.6445 mL | 3.2227 mL | 6.4454 mL | 12.8908 mL | 16.1134 mL |
| 10 mM | 0.3223 mL | 1.6113 mL | 3.2227 mL | 6.4454 mL | 8.0567 mL |
| 50 mM | 0.0645 mL | 0.3223 mL | 0.6445 mL | 1.2891 mL | 1.6113 mL |
| 100 mM | 0.0322 mL | 0.1611 mL | 0.3223 mL | 0.6445 mL | 0.8057 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- (S)-5-Hydroxy-1-(4-hydroxy-3-methoxyphenyl)-7-phenylheptan-3-one
Catalog No.:BCX0508
CAS No.:1220110-76-8
- 1β-Hydroxy-8α-methoxyeremophila-7(11),9-dien-12,8β-olide
Catalog No.:BCX0507
CAS No.:849700-45-4
- Methylconiferin
Catalog No.:BCX0506
CAS No.:883150-46-7
- Iristectorin B
Catalog No.:BCX0505
CAS No.:94396-09-5
- Vavain
Catalog No.:BCX0504
CAS No.:199996-77-5
- 6-Demethoxyirigenin
Catalog No.:BCX0503
CAS No.:1348833-10-2
- Oxyphyllenodiol A
Catalog No.:BCX0502
CAS No.:363610-30-4
- Teuhetenone A
Catalog No.:BCX0501
CAS No.:152481-80-6
- Isocrenatoside
Catalog No.:BCX0500
CAS No.:221895-09-6
- Erythrinin E
Catalog No.:BCX0499
CAS No.:2731101-51-0
- Crenatoside
Catalog No.:BCX0498
CAS No.:61276-16-2
- Curdionolide B
Catalog No.:BCX0497
CAS No.:1190225-68-3
- D-Mannosamine hydrochloride
Catalog No.:BCX0510
CAS No.:5505-63-5
- D-Galactosamine hydrochloride
Catalog No.:BCX0511
CAS No.:1772-03-8
- N-acetyl-D-galactosamine
Catalog No.:BCX0512
CAS No.:1811-31-0
- N-Acetyl-D-mannosamine
Catalog No.:BCX0513
CAS No.:7772-94-3
- Xanthomicrol
Catalog No.:BCX0514
CAS No.:16545-23-6
- 5,7-Dihydroxy-3,8,3',4'-tetramethoxyflavone
Catalog No.:BCX0515
CAS No.:42923-42-2
- 5,4'-Dihydroxy-6,7,8,3'-tetramethoxyflavone
Catalog No.:BCX0516
CAS No.:16520-78-8
- Tetrahydroauroglaucin
Catalog No.:BCX0517
CAS No.:40434-07-9
- Sanggenon F
Catalog No.:BCX0518
CAS No.:85889-03-8
- 4-Hydroxyphenylpyruvic acid
Catalog No.:BCX0519
CAS No.:156-39-8
- Isodihydroauroglaucin
Catalog No.:BCX0520
CAS No.:74886-31-0
- Demethoxysudachitin
Catalog No.:BCX0521
CAS No.:4323-80-2
Metabolomic Profiling and Identification of Antioxidant and Antidiabetic Compounds from Leaves of Different Varieties of Morus alba Linn Grown in Kashmir.[Pubmed:35874221]
ACS Omega. 2022 Jul 8;7(28):24317-24328.
Mulberry (Morus alba L.) is commonly cultivated in Asian countries as a traditional medicine and food supplement. Four Kashmiri Morus alba varieties (Zagtul, Chtattatual, Chattatual Zaingir, and Brentul Kashmir) were evaluated for their proximate composition, mineral content, total phenolic and flavonoid content, antioxidant potential, and antihyperglycemic activity. Furthermore, TLC-MS-bioautography was used for the identification of antioxidant and antidiabetic compounds in the best active extract. Lastly, UPLC-MS was employed for metabolomic profiling of the best variety of M. alba. Among all the varieties, the Zagtul variety was found to have the highest phenolic (71.10 +/- 0.44 mg GAE/g DW) and flavonoid (53.22 +/- 0.69 mg rutin/g DW) content. The highest antioxidant potential (DPPH) with an IC(50) value of 107.88 +/- 3.8 mug/mL was recorded for the Zagtul variety. Similarly, alpha-amylase and alpha-glucosidase inhibition for antidiabetic potential with IC(50) 74.76 +/- 6.76 and 109.19 +/- 5.78 mug/mL, respectively, was recorded in Zagtul variety. TLC-MS-bioautography for identification of bioactive compounds revealed the presence of chlorogenic acid for antioxidant potential and 1-deoxynojirimycin (DNJ) and syringic acid for antidiabetic potential. Further, bioactive compounds responsible for diverse functions of M. alba were confirmed by UPLC-MS in both negative and positive modes. However, major compounds in the Zagtul variety were identified as chlorogenic acid, Moracin N, gallic acid, ferulic acid, morin, 1-deoxynojirimycin, and syringic acid. Hence, based on our findings, it can be concluded that M. alba leaves can be consumed as a promising dietary supplement and can be formulated as phytopharmaceutical for the management of various metabolic disorders.
SWATH-based quantitative proteomic analysis of Morus alba L. leaves after exposure to ultraviolet-B radiation and incubation in the dark.[Pubmed:35429828]
J Photochem Photobiol B. 2022 May;230:112443.
Morus alba is a woody shrub of the family Moraceae and used as traditional Chinese medicine for a long history. Ultraviolet-B (UV-B) radiation, as a kind of abiotic stress factor, affected the growth and secondary metabolism in M. alba. Previous studies indicated that the contents of several secondary metabolites such as Moracin N, chalcomaricin were significantly increased under high level UV-B radiation and dark incubation in M. alba leaves. To reveal the response mechanism under UV-B radiation and dark incubation in M. alba leaves, SWATH-based quantitative proteomic analysis was performed. Totally, 716 proteins were identified and quantified in the control, UVB, and UVD groups. Among them, 123 proteins and 96 proteins were identified as differentially abundant proteins in UVB group and UVD groups, respectively. Proteins related to photosynthesis, amino acid biosynthesis, and tocopherol biosynthesis were significantly altered in UVB group, while proteins related to the biosynthesis of phenolic compounds were significantly altered in UVD group. In addition, the abundances of proteins involved in the ubiquitin-proteasome system (UPS) were significantly increased in both UVB and UVD groups, indicating that UPS combined with secondary mechanism participated in the resistance to UV-B radiation and dark incubation. The obtained results provide novel insight into the effects of high level UV-B radiation on M. alba leaves and on the strategies used for maximizing the chemical constituents and the medicinal value of the M. alba leaves.
Tandem mass tag-based proteomic analysis of endoplasmic reticulum proteins in mulberry leaves under ultraviolet-B and dark stress.[Pubmed:35289407]
Physiol Plant. 2022 Mar;174(2):e13667.
Mulberry leaves have been used in traditional Chinese medicine due to their antioxidant, antidiabetic, and antihyperlipidemic properties. A previous study showed that ultraviolet-B radiation followed by dark incubation could improve the contents of active ingredients in mulberry leaves, such as Moracin N and chalcomoracin. The endoplasmic reticulum (ER) serves as a protein quality control center and the location for protein synthesis, which is involved in the response to the environmental stress in plants. To investigate the mechanisms in response to ultraviolet-B radiation followed by dark incubation (UV + D), ER proteomics was performed on mulberry leaves. The ER protein markers, glucose-regulated protein (GRP78), and calnexin (CNX), were significantly higher in the ER fraction than in the total protein fraction, indicating that the ER was purified. Compared to the control, the abundance of protein disulfide isomerase, UDP-glucose glycoprotein glucosyltransferase, CNX, and calreticulin proteins decreased, while of the abundance of heat shock-related proteins increased under stress. P450 enzyme system-related proteins and ribosomal proteins showed significant increases. These results suggest that under UV + D stress, mulberry leaves activated the cell redox and ER quality control systems, enhancing protein synthesis and weakening N-glycan biosynthesis in the ER to resist the damage.
Identification of prenylated phenolics in mulberry leaf and their neuroprotective activity.[Pubmed:34281775]
Phytomedicine. 2021 Sep;90:153641.
BACKGROUND: Neurodegenerative diseases are becoming increasingly prevalent over the world. Therefore, drug development in this field is urgently required. Neuron impairment leads to the pathogenesis of neurodegenerative diseases, while amelioration of oxidative stress can inhibit the impairment. As a traditional Chinese medicine, mulberry leaf exhibits various pharmacological properties, including neuroprotective activity. But the major components responsible for the neuroprotective activity of mulberry leaf remained unknown. Phytochemicals were potent candidates of neuroprotective drug. Prenylated phenolics are the leading phytochemicals present in mulberry leaf. PURPOSE: The aim of this study was to investigate the neuroprotective activities and mechanisms of prenylated phenolics. METHODS: The chemical structure of isolated compounds were elucidated by MS and NMR. UPLC-MS/MS was used to determine the contents of prenylated phenolics in fresh mulberry leaf. Neurotoxicity was induced by erastin in HT22 cells. CCK-8 assay was performed to assess cell viability. ROS production, GSH level and iron release were monitored by using DCFH-DA, monobromobimane, and FeRhoNox-1, respectively. qRT-PCR and Western blotting assays were performed to assess gene and protein expression, respectively. RESULTS: Four prenylated phenolics, including isobavachalcone, morachalcone B, Moracin N and morachalcone A were isolated and identified from mulberry leaf. Their levels in fresh mulberry leaf were in a decreasing order, Moracin N > morachalcone A > morachalcone B > isobavachalcone. Moreover, Moracin N showed a good neuroprotective activity with an EC(50) < 0.50 microM. The neuroprotective mechanisms of Moracin N included inhibition of glutathione depletion, glutathione peroxidase 4 (GPx4) inactivation, reactive oxygen species (ROS) overproduction and iron accumulation, as well as improvement of intracellular antioxidant enzyme activities. Moracin N augmented the transcriptional levels of genes involved in antioxidant defense and glutathione biosynthesis in the early state of ferroptosis induction, and downregulated expression of genes related to iron accumulation and lipid peroxidation. CONCLUSION: The results confirmed that Moracin N was a good ferroptosis inhibitor, which exerted neuroprotective activity through preventing from oxidative stress.


