Tetrahydropalmatine HydrochlorideCAS# 6024-85-7 |
Quality Control & MSDS
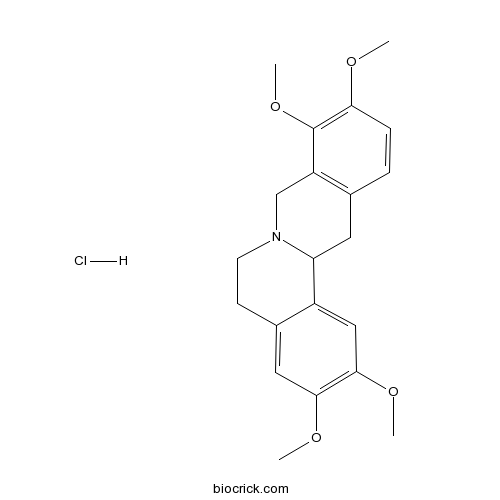
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 6024-85-7 | SDF | Download SDF |
| PubChem ID | 6602555 | Appearance | White crystalline powder |
| Formula | C21H26ClNO4 | M.Wt | 391.89 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 2,3,9,10-tetramethoxy-6,8,13,13a-tetrahydro-5H-isoquinolino[2,1-b]isoquinoline;hydrochloride | ||
| SMILES | COC1=C(C2=C(CC3C4=CC(=C(C=C4CCN3C2)OC)OC)C=C1)OC.Cl | ||
| Standard InChIKey | MGSZZQQRTPWMEI-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C21H25NO4.ClH/c1-23-18-6-5-13-9-17-15-11-20(25-3)19(24-2)10-14(15)7-8-22(17)12-16(13)21(18)26-4;/h5-6,10-11,17H,7-9,12H2,1-4H3;1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Tetrahydropalmatine Hydrochloride Dilution Calculator

Tetrahydropalmatine Hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5517 mL | 12.7587 mL | 25.5174 mL | 51.0347 mL | 63.7934 mL |
| 5 mM | 0.5103 mL | 2.5517 mL | 5.1035 mL | 10.2069 mL | 12.7587 mL |
| 10 mM | 0.2552 mL | 1.2759 mL | 2.5517 mL | 5.1035 mL | 6.3793 mL |
| 50 mM | 0.051 mL | 0.2552 mL | 0.5103 mL | 1.0207 mL | 1.2759 mL |
| 100 mM | 0.0255 mL | 0.1276 mL | 0.2552 mL | 0.5103 mL | 0.6379 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- AZD-5438
Catalog No.:BCC3689
CAS No.:602306-29-6
- Oleanolic acid-3-O-beta-D-glucopyranosyl (1→2)-alpha-L-arabinopyranoside
Catalog No.:BCN1406
CAS No.:60213-69-6
- Altamycin A
Catalog No.:BCN1823
CAS No.:60202-22-4
- Coptisine chloride
Catalog No.:BCN6321
CAS No.:6020-18-4
- 1,2-dihydroxy-3-methyl-anthracene-9,10-dione
Catalog No.:BCN1404
CAS No.:602-63-1
- Thiocolchicoside
Catalog No.:BCN8442
CAS No.:602-41-5
- Taspine
Catalog No.:BCN6956
CAS No.:602-07-3
- Licoflavonol
Catalog No.:BCN6828
CAS No.:60197-60-6
- Methyl 15-hydroxy-7-oxodehydroabietate
Catalog No.:BCN7674
CAS No.:60188-95-6
- Corypalmine
Catalog No.:BCN4111
CAS No.:6018-40-2
- Arecaidine hydrochloride
Catalog No.:BCN8530
CAS No.:6018-28-6
- Sodium 4-Aminosalicylate
Catalog No.:BCC4609
CAS No.:6018-19-5
- (±)-Nipecotic acid
Catalog No.:BCC6576
CAS No.:60252-41-7
- Ethyl(1-hydroxy-4-oxocyclohexa-2,5-dien-1-yl)acetate
Catalog No.:BCN1405
CAS No.:60263-06-1
- H-D-HoSer-OH
Catalog No.:BCC3243
CAS No.:6027-21-0
- Aspalathin
Catalog No.:BCC8122
CAS No.:6027-43-6
- Guanosine-2'(3')-monophosphate disodium salt
Catalog No.:BCC3608
CAS No.:6027-83-4
- Guvacine hydrochloride
Catalog No.:BCC6574
CAS No.:6027-91-4
- Gestodene
Catalog No.:BCC4490
CAS No.:60282-87-3
- 7-Angeloylretronecine
Catalog No.:BCN2036
CAS No.:6029-82-9
- Rinderine
Catalog No.:BCN1971
CAS No.:6029-84-1
- Latifoline
Catalog No.:BCN1978
CAS No.:6029-86-3
- Fulvine
Catalog No.:BCN2082
CAS No.:6029-87-4
- Auriculasin
Catalog No.:BCN3970
CAS No.:60297-37-2
The inhibitory effect of levo-tetrahydropalmatine on the methamphetamine-induced spatial memory impairment in mice.[Pubmed:29447954]
Neurosci Lett. 2018 Apr 13;672:34-39.
Methamphetamine (METH) administration results in addiction and memory impairment. Previous studies have suggested that levo-tetrahydropalmatine (l-THP), an alkaloid purified from the Chinese herb Corydalis, attenuates the behavioral changes induced by METH. Therefore, in this study, we explored whether l-THP could also protect against the METH-induced memory impairment examined using the Morris water maze (MWM). We found that low dose of METH (1.0mg/kg) treated for 20 consecutive days prior to the MWM experiment impaired spatial memory retention but not acquisition in mice. In addition, high dose of METH (10.0mg/kg) treated during the spatial learning phase for five consecutive days impaired both the acquisition and retention of spatial memory. Moreover, both of these impairments induced by METH were reversed by l-THP treatment, indicating a potential protective role of l-THP in METH use.
Protective effects of tetrahydropalmatine against ketamine-induced learning and memory injury via antioxidative, anti-inflammatory and anti-apoptotic mechanisms in mice.[Pubmed:29512789]
Mol Med Rep. 2018 May;17(5):6873-6880.
Tetrahydropalmatine exerts numerous pharmacological activities, including analgesic and narcotic effects; anti-arrhythmic, blood pressure lowering and cardioprotective effects; protective effects against cerebral ischemia-reperfusion injury; inhibition of platelet aggregation; prevention of ulcerative diseases and inhibition of gastric acid secretion; antitumor effects; and beneficial effects on the withdrawal symptoms associated with drug addiction. The present study aimed to investigate the protective effects of tetrahydropalmatine against ketamineinduced learning and memory impairment in mice. The Morris water maze test and open field test were used to analyzed learning and memory impairment in mice. ELISA kits and western blotting were used to analyze oxidative stress, inflammation factors, caspease3 and caspase9, iNOS, glial fibrillary acidic protein (GFAP), glial cellderived neurotrophic factor (GDNF), cytochrome c and phospholipase C (PLC)gamma1 protein expression. The results demonstrated that tetrahydropalmatine treatment significantly decreased escape latency in the learning phase and increased the number of platform site crossings in ketamineinduced mice. In addition, tetrahydropalmatine significantly inhibited oxidative stress, inflammation and acetylcholinesterase activity, and decreased acetylcholine levels in ketamineinduced mice. Tetrahydropalmatine also suppressed iNOS protein expression, weakened caspase3 and caspase9 activation, inhibited nuclear factorkappaB, glial fibrillary acidic protein, cytochrome c and phospholipase Cgamma1 protein expression, and induced glial cellderived neurotrophic factor protein expression in ketamineinduced mice. Taken together, these results indicated that tetrahydropalmatine may protect against ketamineinduced learning and memory impairment in mice via antioxidative, antiinflammatory and antiapoptotic mechanisms. The present study provided an experimental basis for the clinical application of tetrahydropalmatine to reduce the severe side effects associated with ketamine therapy in future studies.
Alkaloids from Corydalis decumbens suppress neuronal excitability in primary cultures of mouse neocortical neurons.[Pubmed:29571149]
Phytochemistry. 2018 Jun;150:85-92.
Eight previously undescribed alkaloids, named corydemine, dihydrocorydemine, corydedine, 8,13-dioxo-14-hydroxytetrahydropalmatine, egenine-alpha-N-oxide, egenine-beta-N-oxide, 7'-O-ethylegenine-alpha-N-oxide, and 7'-O-ethylegenine-beta-N-oxide, together with three known ones, muramine, l-tetrahydropalmatine, and (+)-egenine, were isolated from the bulbs of Corydalis decumbens. Their structures were elucidated by comprehensive spectroscopic analysis and chemical correlation. The isolated compounds were tested for their ability to modulate neuronal excitability in primary cultured neocortical neurons. Four of the compounds, corydemine, dihydrocorydemine, muramine, and l-tetrahydropalmatine, inhibited neuronal excitability with IC50 values of 3.6, 16.7, 13.5 and 14.0muM, respectively.
Levo-Tetrahydropalmatine Attenuates Progression of Abdominal Aortic Aneurysm in an Elastase Perfusion Rat Model via Suppression of Matrix Metalloproteinase and Monocyte Chemotactic Protein-1.[Pubmed:29388563]
Med Sci Monit. 2018 Feb 1;24:652-660.
BACKGROUND Levo-tetrahydropalmatine (L-THP) is a tetrahydro protoberberine isoquinoline alkaloid obtained from the genera Stephania and Corydalis. In the present research, we evaluated the effects of L-THP on the progression of aortic aneurysms (AAs) in experimental rats induced with perfusion of elastase. MATERIAL AND METHODS Thirty-six Sprague-Dawley rats were divided into sham-operated, control, and L-THP treated groups (n=12 in each group). The rats in the control group and the L-THP group received intra-aortic perfusion of elastase to induce AAs; the sham-operated group received perfusion of saline. The rats in the L-THP group received a dose of 15 mg/kg/day, the control and the sham group received saline treatment. The animals were evaluated for aortic diameters (ADs) and systolic blood pressure (SBP) just before and after the elastase perfusion, and 24 days after perfusion. The extracts of the aortas were evaluated by western blotting and immunohistochemistry. RESULTS In the control group, a significant increase in aortic size was observed (p<0.05) compared to the sham group after 24 days post-perfusion, whereas the L-THP group showed a decrease in diameter compared to the control group (p<0.05). The SBP increased significantly in the control group compared to the sham group. The L-THP group showed reduction in SBP, exhibited decreased expression of metalloproteinase and monocyte chemotactic protein-1, and the tissue samples also exhibited significant decreased levels of iNOS compared to the control group. L-THP treatment prevented loss of vascular smooth muscle cells (VSMCs) of the aortic walls. CONCLUSIONS L-THP inhibited progression of AAs in rats by curbing inflammation, oxidative stress, and conserving VSMCs, suggesting a new therapeutic approach for managing AAs.
Analysis of isoquinoline alkaloids from Mahonia leschenaultia and Mahonia napaulensis roots using UHPLC-Orbitrap-MS(n) and UHPLC-QqQLIT-MS/MS.[Pubmed:29404021]
J Pharm Anal. 2017 Apr;7(2):77-86.
Mahonia leschenaultia (ML) and Mahonia napaulensis (MN) are less known and unexplored medicinal plants of the family Berberidaceae. They are used by the Todas of Nilgiris in their religious and medical practices but chemically less identified. Hence, we decided to do extensive phytochemical analysis to explore the potential of these plant extracts. An ultrahigh performance electrospray tandem mass spectrometry (UHPLC-ESI-MS/MS) method was successfully developed for qualitative analysis of the bioactive components in Mahonia species using Orbitrap Velos Pro mass spectrometer. Sixteen compounds were identified by comparison of their retention times and mass spectra (MS) with authentic standards and reported literature. Multi-stage mass spectra (MS(2-8)) for the identification of protoberberine and aporphine alkaloids showed the sequential expulsion of all the substituents attached with their basic skeleton followed by CO loss. Eight of the identified compounds (berberine, jatrorrhizine, palmatine, magnoflorine, isocorydine, glaucine, tetrahydropalmatine and tetrahydroberberine) were simultaneously determined by another UHPLC-ESI-MS/MS method under the multiple reactions monitoring (MRM) mode quantitatively using triple quadrupole linear ion trap mass spectrometer. The analytical method was validated for 8 bioactive compounds with overall recovery in the range 98.5%-103.6% (RSD/=0.9995) over the concentration range of 0.5-1000 ng/mL and successfully applied in ML and MN roots, which suggests the suitability of the proposed approach for the routine analysis of Mahonia species and their quality control.
Development and Validation of a HPLC-ESI-MS/MS Method for Simultaneous Quantification of Fourteen Alkaloids in Mouse Plasma after Oral Administration of the Extract of Corydalis yanhusuo Tuber: Application to Pharmacokinetic Study.[Pubmed:29561801]
Molecules. 2018 Mar 21;23(4). pii: molecules23040714.
The tuber of Corydalis yanhusuo is a famous traditional Chinese medicine and found to have potent pharmacological effects, such as antinociceptive, antitumor, antibacterial, anti-inflammatory, and anti-depressive activities. Although there are several methods to be developed for the analysis and detection of the bioactive ingredients' alkaloids, so far, only few prominent alkaloids could be quantified, and in vitro and in vivo changes of comprehensive alkaloids after oral administration are still little known. In this study, we first developed a simple and sensitive high-performance liquid chromatography-electrospray ionization-tandem mass spectrometry (HPLC-ESI-MS/MS) method to quantify the comprehensive alkaloids of extracts of C. yanhusuo in mouse plasma, using nitidine chloride as an internal standard. As results, at least fourteen alkaloids, including an aporphine (oxoglaucine), a protopine (protopine), five tertiary alkaloids (corydaline, tetrahydroberberine, tetrahydropalmatine, tetrahydrocolumbamine, and tetrahydrocoptisine) and seven quaternary alkaloids (columbamine, palmatine, berberine, epiberberine, coptisine, jatrorrhizine, and dehydrocorydaline) could be well quantified simultaneously in mouse plasma. The lower limits of quantification were greater than, or equal to, 0.67 ng/mL, and the average matrix effects ranged from 96.4% to 114.3%. The mean extraction recoveries of quality control samples were over 71.40%, and the precision and accuracy were within the acceptable limits. All the analytes were shown to be stable under different storage conditions. Then the established method was successfully applied to investigate the pharmacokinetics of these alkaloids after oral administration of the extract of Corydalis yanhusuo in mice. To the best of our knowledge, this is the first document to report the comprehensive and simultaneous analyses of alkaloids of C. yanhusuo in mouse plasma. It was efficient and useful for comprehensive pharmacokinetic and metabolomic analyses of these complex alkaloids after drug administration.


