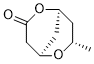
TetraketideCAS# 276869-83-1 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 276869-83-1 | SDF | Download SDF |
| PubChem ID | N/A | Appearance | Powder |
| Formula | C8H12O3 | M.Wt | 156.2 |
| Type of Compound | Other NPs | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Tetraketide Dilution Calculator

Tetraketide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 6.402 mL | 32.0102 mL | 64.0205 mL | 128.041 mL | 160.0512 mL |
| 5 mM | 1.2804 mL | 6.402 mL | 12.8041 mL | 25.6082 mL | 32.0102 mL |
| 10 mM | 0.6402 mL | 3.201 mL | 6.402 mL | 12.8041 mL | 16.0051 mL |
| 50 mM | 0.128 mL | 0.6402 mL | 1.2804 mL | 2.5608 mL | 3.201 mL |
| 100 mM | 0.064 mL | 0.3201 mL | 0.6402 mL | 1.2804 mL | 1.6005 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Tatsiensine
Catalog No.:BCN0428
CAS No.:86695-18-3
- Hyperwightin C
Catalog No.:BCN0427
CAS No.:2374723-87-0
- Hyperwightin D
Catalog No.:BCN0426
CAS No.:2374723-88-1
- Hyperwightin E
Catalog No.:BCN0425
CAS No.:2374723-89-2
- Hyperwightin B
Catalog No.:BCN0424
CAS No.:2374723-86-9
- Deacetyltatsiensine
Catalog No.:BCN0423
CAS No.:86695-19-4
- Acrofolione A
Catalog No.:BCN0422
CAS No.:820975-61-9
- 8-Oxoeremophil-6-en-12-oic aicd
Catalog No.:BCN0421
CAS No.:1313436-97-3
- Acetyldihydromicromelin B
Catalog No.:BCN0420
CAS No.:1427351-73-2
- (3R)-Hydroxyibogaine
Catalog No.:BCN0419
CAS No.:2276636-13-4
- 23-Hydroxyursolic acid
Catalog No.:BCN0418
CAS No.:94414-19-4
- 1-O-Caffeoylglucose
Catalog No.:BCN0417
CAS No.:14364-08-0
- Dehydro-γ-tocopherol
Catalog No.:BCN0430
CAS No.:96273-97-1
- n-Butyl orsellinate
Catalog No.:BCN0431
CAS No.:31489-30-2
- Euscapholide
Catalog No.:BCN0432
CAS No.:220862-15-7
- Acrofolione B
Catalog No.:BCN0433
CAS No.:820975-62-0
- Gnetifolin A
Catalog No.:BCN0434
CAS No.:137476-72-3
- Siwanine B
Catalog No.:BCN0435
CAS No.:159903-63-6
- Tuberostemonine D
Catalog No.:BCN0436
CAS No.:1627827-84-2
- 19-Hydroxypseudovincadifformine
Catalog No.:BCN0437
CAS No.:2340171-73-3
- Genipin 10-O-glucoside
Catalog No.:BCN0438
CAS No.:1947317-95-4
- Cyclobutanedichalcone
Catalog No.:BCN0439
CAS No.:861706-38-9
- Farfugin A
Catalog No.:BCN0440
CAS No.:36061-18-4
- Acrovestenol
Catalog No.:BCN0441
CAS No.:864679-69-6
Tetraketide alpha-pyrone reductases in sporopollenin synthesis pathway in Gerbera hybrida: diversification of the minor function.[Pubmed:34593769]
Hortic Res. 2021 Oct 1;8(1):207.
The structurally robust biopolymer sporopollenin is the major constituent of the exine layer of pollen wall and plays a vital role in plant reproductive success. The sporopollenin precursors are synthesized through an ancient polyketide biosynthetic pathway consisting of a series of anther-specific enzymes that are widely present in all land plant lineages. Tetraketide alpha-pyrone reductase 1 (TKPR1) and TKPR2 are two reductases catalyzing the final reduction of the carbonyl group of the polyketide synthase-synthesized Tetraketide intermediates to hydroxylated alpha-pyrone compounds, important precursors of sporopollenin. In contrast to the functional conservation of many sporopollenin biosynthesis associated genes confirmed in diverse plant species, TKPR2's role has been addressed only in Arabidopsis, where it plays a minor role in sporopollenin biosynthesis. We identified in gerbera two non-anther-specific orthologues of AtTKPR2, Gerbera reductase 1 (GRED1) and GRED2. Their dramatically expanded expression pattern implies involvement in pathways outside of the sporopollenin pathway. In this study, we show that GRED1 and GRED2 are still involved in sporopollenin biosynthesis with a similar secondary role as AtTKPR2 in Arabidopsis. We further show that this secondary role does not relate to the promoter of the gene, AtTKPR2 cannot rescue pollen development in Arabidopsis even when controlled by the AtTKPR1 promoter. We also identified the gerbera orthologue of AtTKPR1, GTKPR1, and characterized its crucial role in gerbera pollen development. GTKPR1 is the predominant TKPR in gerbera pollen wall formation, in contrast to the minor roles GRED1 and GRED2. GTKPR1 is in fact an excellent target for engineering male-sterile gerbera cultivars in horticultural plant breeding.
Identification and Characterization of a New Type III Polyketide Synthase from a Marine Yeast, Naganishia uzbekistanensis.[Pubmed:33322429]
Mar Drugs. 2020 Dec 11;18(12). pii: md18120637.
A putative Type III Polyketide synthase (PKSIII) encoding gene was identified from a marine yeast, Naganishia uzbekistanensis strain Mo29 (UBOCC-A-208024) (formerly named as Cryptococcus sp.) isolated from deep-sea hydrothermal vents. This gene is part of a distinct phylogenetic branch compared to all known terrestrial fungal sequences. This new gene encodes a C-terminus extension of 74 amino acids compared to other known PKSIII proteins like Neurospora crassa. Full-length and reduced versions of this PKSIII were successfully cloned and overexpressed in a bacterial host, Escherichia coli BL21 (DE3). Both proteins showed the same activity, suggesting that additional amino acid residues at the C-terminus are probably not required for biochemical functions. We demonstrated by LC-ESI-MS/MS that these two recombinant PKSIII proteins could only produce tri- and Tetraketide pyrones and alkylresorcinols using only long fatty acid chain from C8 to C16 acyl-CoAs as starter units, in presence of malonyl-CoA. In addition, we showed that some of these molecules exhibit cytotoxic activities against several cancer cell lines.
The chemistry and biology of fungal meroterpenoids (2009-2019).[Pubmed:33320161]
Org Biomol Chem. 2021 Mar 4;19(8):1644-1704.
Fungal meroterpenoids are secondary metabolites from mixed terpene-biosynthetic origins. Their intriguing chemical structural diversification and complexity, potential bioactivities, and pharmacological significance make them attractive targets in natural product chemistry, organic synthesis, and biosynthesis. This review provides a systematic overview of the isolation, chemical structural features, biological activities, and fungal biodiversity of 1585 novel meroterpenoids from 79 genera terrestrial and marine-derived fungi including macrofungi, Basidiomycetes, in 441 research papers in 2009-2019. Based on the nonterpenoid starting moiety in their biosynthesis pathway, meroterpenoids were classified into four categories (polyketide-terpenoid, indole-, shikimate-, and miscellaneous-) with polyketide-terpenoids (mainly Tetraketide-) and shikimate-terpenoids as the primary source. Basidiomycota produced 37.5% of meroterpenoids, mostly shikimate-terpenoids. The genera of Ganoderma, Penicillium, Aspergillus, and Stachybotrys are the four dominant producers. Moreover, about 56% of meroterpenoids display various pronounced bioactivities, including cytotoxicity, enzyme inhibition, antibacterial, anti-inflammatory, antiviral, antifungal activities. It's exciting that several meroterpenoids including antroquinonol and 4-acetyl antroquinonol B were developed into phase II clinically used drugs. We assume that the chemical diversity and therapeutic potential of these fungal meroterpenoids will provide biologists and medicinal chemists with a large promising sustainable treasure-trove for drug discovery.
The developmental dynamics of the sweet sorghum root transcriptome elucidate the differentiation of apoplastic barriers.[Pubmed:32024414]
Plant Signal Behav. 2020 Mar 3;15(3):1724465.
Apoplastic barriers in the endodermis, such as Casparian strips and suberin lamellae, control the passage of water and minerals into the stele. Apoplastic barriers are thus thought to contribute to salt exclusion in salt-excluding plants such as sweet sorghum (Sorghum bicolor). However, little is known about the genes involved in the development of the apoplastic barrier. Here, we identified candidate genes involved in Casparian strip and suberin lamella development in the roots of a sweet sorghum line (M-81E). Three distinct developmental regions (no differentiation, developing, and mature) were identified based on Casparian strip and suberin lamella staining in root cross sections. Sequencing of RNA extracted from these distinct sections identified key genes participating in the differentiation of the apoplastic barrier. The different sections were structurally distinct, presumably due to differences in gene expression. Genes controlling the phenylpropanoid pathway, fatty acid elongation, and fatty acid omega-hydroxylation appeared to be directly responsible for the formation of the apoplastic barrier. Our dataset elucidates the molecular processes underpinning apoplastic barrier development and provides a basis for future research on molecular mechanisms of apoplastic barrier formation and salt exclusion.Abbreviations: SHR, SHORTROOT; MYB, MYB DOMAIN PROTEIN; CIFs, Casparian strip integrity factors; CASP, Casparian strip domain proteins; PER, peroxidase; ESB1, ENHANCED SUBERIN1; CS, Casparian strip; RPKM, reads per kilobase per million reads; DEGs, differentially expressed genes; FDR, false discovery rate; GO, Gene Ontology; KEGG, Kyoto Encyclopedia of Genes and Genomes; RNA-seq, RNA sequencing; PAL, phenylalanine ammonia-lyase; CYP, cytochrome P450 monooxygenases; 4CL, 4-coumarate-CoA ligase; AAE5, ACYL-ACTIVATING ENZYME5; CCR, cinnamoyl CoA reductase; TKPR, Tetraketide ALPHA-PYRONE REDUCTASE1; CAD, cinnamyl alcohol dehydrogenase; HST, shikimate O-hydroxycinnamoyltransferase; PMAT2, PHENOLIC GLUCOSIDE MALONYLTRANSFERASE2; CCOAOMT, caffeoyl-CoA O-methyltransferase; KCS, beta-ketoacyl-CoA synthase; CUT1, CUTICULAR PROTEIN1; DET2, 5-alpha-reductase; TAX, 3'-N-debenzoyl-2'-deoxytaxol N-benzoyltransferase; CER1, ECERIFERUM1; FAR, fatty acyl reductase; AF-CoA, alcohol-forming fatty acyl-CoA reductase; ABCG, ATP-binding cassette, subfamily G; ERF, ethylene-responsive transcription factor; HSF, heat stress transcription factor; NTF, NUCLEAR TRANSCRIPTION FACTOR Y SUBUNIT B-5; GPAT, glycerol 3-phosphate acyltransferase.
Demystifying and unravelling the molecular structure of the biopolymer sporopollenin.[Pubmed:32003875]
Rapid Commun Mass Spectrom. 2020 May 30;34(10):e8740.
RATIONALE: We report the unsolved molecular structure of the complex biopolymer sporopollenin exine extracted from Lycopodium clavatum pollen grains. METHODS: TOF-SIMS and CID-MS/MS, MALDI-TOF-MS and CID-TOF/TOF-MS/MS were used for the analysis of this complex biopolymer sporopollenin exine extracted from Lycopodium clavatum pollen grains. Solid-state (1) H- and (13) C-NMR, 2D (1) H-(1) H NOESY, Rotor-synchronized (13) C{(1) H} HSQC, and (13) C{(1) H} multi CP-MAS NMR experiments were used to confirm the structural assigments revealed by MS and MS/MS studies. Finally, high-resolution XPS was used to check for the presence of aromatic components in sporopollenin. RESULTS: The combined MS and NMR analyses showed that sporopollenin contained poly(hydroxy acid) dendrimer-like networks with glycerol as a core unit, which accounted for the sporopollenin empirical formula. In addition, these analyses showed that the hydroxy acid monomers forming this network contained a beta-diketone moiety. Moreover, MALDI-TOF-MS and MS/MS allowed us to identify a unique macrocyclic oligomeric unit composed of polyhydroxylated Tetraketide-like monomers. Lastly, high-resolution X-ray photoelectron spectroscopy (HR-XPS) showed the absence of aromaticity in sporopollenin. CONCLUSIONS: We report for the first time the two main building units that form the Lycopodium clavatum sporopollenin exine. The first building unit is a macrocyclic oligomer and/or polymer composed of polyhydroxylated Tetraketide-like monomeric units, which represents the main rigid backbone of the sporopollenin biopolymer. The second building unit is the poly(hydroxy acid) network in which the hydroxyl end groups can be covalently attached by ether links to the hydroxylated macrocyclic backbone to form the sporopollenin biopolymer, a spherical dendrimer. Such spherical dendrimers are a typical type of microcapsule that have been used for drug delivery applications. Finally, HR-XPS indicated the total absence of aromaticity in the sporopollenin exine.
Structure of the Cannabis sativa olivetol-producing enzyme reveals cyclization plasticity in type III polyketide synthases.[Pubmed:31605668]
FEBS J. 2020 Apr;287(8):1511-1524.
In the native pathway to therapeutic cannabinoid biosynthesis in Cannabis sativa, the three-step production of a key intermediate, olivetolic acid, is catalysed by the enzymes Tetraketide synthase (TKS; linear Tetraketide intermediate production in two stages) and olivetolic acid cyclase (OAC; final C2 --> C7 aldol condensation). In the absence of OAC, a nonenzymatic C2 --> C7 decarboxylative aldol condensation of the Tetraketide intermediate occurs forming olivetol. TKS is a type III polyketide synthase, and the question arises why it is unable to form olivetolic acid directly, but instead forms this unwanted side product. We determined the TKS, CoA complex structure, and performed structurally guided mutagenesis studies to identify potential residues responsible for cyclization pathway discrimination in type III polyketide synthases. Prior studies suggested an 'aldol switch' is necessary to allow linear Tetraketide intermediate release prior to cyclization, thereby enabling subsequent olivetolic acid production by OAC. However, our studies do not support the presence of a universal or predictable 'aldol switch' consensus sequence. Instead, we propose the mode of ordered active site water activation between type III polyketide synthases catalysing different cyclization mechanisms is subtle and homologue-specific. Our work indicates that subtle structural variations between homologous enzymes can have a major mechanistic impact on the catalytic outcome. This highlights the importance of embedding high-resolution structural analysis of multiple enzyme homologues with classical site-directed mutagenesis studies when investigating highly similar enzymes with different mechanistic pathway outcomes. ENZYMES: TKS, EC 2.3.1.206; OAC, EC 4.4.1.26; chalcone synthase, EC 2.3.1.74; stilbene synthase, EC 2.3.1.95; 2-PS, EC 2.3.1.-. ACCESSION NUMBERS: The atomic coordinates and structure factors for the crystal structure of TKS have been deposited in the Protein Data Bank with accession number 6GW3.
Characterization of an Orphan Type III Polyketide Synthase Conserved in Uncultivated "Entotheonella" Sponge Symbionts.[Pubmed:31430416]
Chembiochem. 2020 Feb 17;21(4):564-571.
Uncultivated bacterial symbionts from the candidate genus "Entotheonella" have been shown to produce diverse natural products previously attributed to their sponge hosts. In addition to these known compounds, "Entotheonella" genomes contain rich sets of biosynthetic gene clusters that lack identified natural products. Among these is a small type III polyketide synthase (PKS) cluster, one of only three clusters present in all known "Entotheonella" genomes. This conserved "Entotheonella" PKS (cep) cluster encodes the type III PKS CepA and the putative methyltransferase CepB. Herein, the characterization of CepA as an enzyme involved in phenolic lipid biosynthesis is reported. In vitro analysis showed a specificity for alkyl starter substrates and the production of tri- and Tetraketide pyrones and Tetraketide resorcinols. The conserved distribution of the cep cluster suggests an important role for the phenolic lipid polyketides produced in "Entotheonella" variants.
Ostkpr1 functions in anther cuticle development and pollen wall formation in rice.[Pubmed:30885140]
BMC Plant Biol. 2019 Mar 18;19(1):104.
BACKGROUND: During pollen wall formation in flowering plants, a conserved metabolon consisting of acyl-CoA synthetase (ACOS), polyketide synthase (PKS) and Tetraketide alpha-pyrone reductase (TKPR), is required for sporopollenin synthesis. Despite this, the precise function of each of these components in different species remains unclear. RESULTS: In this study, we characterized the function of OsTKPR1, a rice orthologue of Arabidopsis TKPR1. Loss of function of OsTKPR1 delayed tapetum degradation, reduced the levels of anther cuticular lipids, and impaired Ubisch body and pollen exine formation, resulting in complete male sterility. In addition, the phenylpropanoid pathway in mutant anthers was remarkably altered. Localization studies suggest that OsTKPR1 accumulates in the endoplasmic reticulum, while specific accumulation of OsTKPR1 mRNA in the anther tapetum and microspores is consistent with its function in anther and pollen wall development. CONCLUSIONS: Our results show that OsTKPR1 is indispensable for anther cuticle development and pollen wall formation in rice, providing new insights into the biochemical mechanisms of the conserved sporopollenin metabolon in flowering plants.
Biosynthetic studies of novel polyketides from the marine sponge-derived fungus Stachylidium sp. 293K04.[Pubmed:30785154]
Org Biomol Chem. 2019 Mar 6;17(10):2747-2752.
The marilones and marilines are a new family of polyketide metabolites recently isolated from the marine-derived fungus Stachylidium sp. 293K04. They are characterized by a phthalide and phthalimidine skeleton, respectively. From a series of feeding experiments using 13C-labeled precursors we could outline the biogenetic origin of the basic carbon skeleton of marilone A and mariline B. Results established the Tetraketide nature of the phthalide/phthalimidine nucleus including the involvement of a methylated acetate starter unit in polyketide biosynthesis, which is unique amongst fungal metabolites.
Investigations into the biosynthesis of the antifungal strobilurins.[Pubmed:30027987]
Org Biomol Chem. 2018 Aug 1;16(30):5524-5532.
The strobilurins are important antifungal metabolites isolated from a number of basidiomycetes and have been valuable leads for the development of commercially important fungicides. Isotopic labelling studies with early and advanced intermediates confirm for the first time that they are produced via a linear Tetraketide, primed with the rare benzoate starter unit, itself derived from phenylalanine via cinnamate. Isolation of a novel biphenyl metabolite, pseudostrobilurin B, provides evidence for the involvement of an epoxide in the key rearrangement to form the beta-methoxyacrylate moiety essential for biological activity. Formation of two bolineol related metabolites, strobilurins Y and Z, also probably involves epoxide intermediates. Time course studies indicate a likely biosynthetic pathway from strobilurin A, with the simplest non-subsubstituted benzoate ring, to strobilurin G with a complex dioxepin terpenoid-derived substituent. Precursor-directed biosynthetic studies allow production of a number of novel ring-halogenated analogues as well as a new pyridyl strobilurin. These studies also provide evidence for a non-linear biosynthetic relationship between strobilurin A and strobilurin B.
The Regulation of Sporopollenin Biosynthesis Genes for Rapid Pollen Wall Formation.[Pubmed:30018171]
Plant Physiol. 2018 Sep;178(1):283-294.
Sporopollenin is the major component of the outer pollen wall (sexine). It is synthesized using a pathway of approximately eight genes in Arabidopsis (Arabidopsis thaliana). MALE STERILITY188 (MS188) and its direct upstream regulator ABORTED MICROSPORES (AMS) are two transcription factors essential for tapetum development. Here, we show that all the sporopollenin biosynthesis proteins are specifically expressed in the tapetum and are secreted into anther locules. MS188, a MYB transcription factor expressed in the tapetum, directly regulates the expression of POLYKETIDE SYNTHASE A (PKSA), PKSB, MALE STERILE2 (MS2), and a CYTOCHROME P450 gene (CYP703A2). By contrast, the expression of CYP704B1, ACYL-COA SYNTHETASE5 (ACOS5), Tetraketide a-PYRONE REDUCTASE1 (TKPR1) and TKPR2 are significantly reduced in ams mutants but not affected in ms188 mutants. However, MS188 but not AMS can activate the expression of CYP704B1, ACOS5, and TKPR1 In ms188, dominant suppression of MS188 homologs reduced the expression of these genes, suggesting that MS188 and other MYB family members play redundant roles in activating their expression. The expression of some sporopollenin synthesis genes (PKSA, PKSB, TKPR2, CYP704B1, and ACOS5) was rescued when MS188 was expressed in ams Therefore, MS188 is a key regulator for activation of sporopollenin synthesis, and AMS and MS188 may form a feed-forward loop that activates the expression of the sporopollenin biosynthesis pathway for rapid pollen wall formation.
New Insights on Cyclization Specificity of Fungal Type III Polyketide Synthase, PKSIIINc in Neurospora crassa.[Pubmed:30013270]
Indian J Microbiol. 2018 Sep;58(3):268-277.
Type III polyketide synthases (PKSs) biosynthesize varied classes of metabolites with diverse bio-functionalities. Inherent promiscuous substrate specificity, multiple elongations of reaction intermediates and several modes of ring-closure, confer the proteins with the ability to generate unique scaffolds from limited substrate pools. Structural studies have identified crucial amino acid residues that dictate type III PKS functioning, though cyclization specific residues need further investigation. PKSIIINc, a functionally and structurally characterized type III PKS from the fungus, Neurospora crassa, is known to biosynthesize alkyl-resorcinol, alkyl-triketide- and alkyl-Tetraketide-alpha-pyrone products. In this study, we attempted to identify residue positions governing cyclization specificity in PKSIIINc through comparative structural analysis. Structural comparisons with other type III PKSs revealed a motif with conserved hydroxyl/thiol groups that could dictate PKSIIINc catalysis. Site-directed mutagenesis of Cys120 and Ser186 to Ser and Cys, respectively, altered product profiles of mutant proteins. While both C120S and S186C proteins retained wild-type PKSIIINc product activity, S186C favoured lactonization and yielded higher amounts of the alpha-pyrone products. Notably, C120S gained new cyclization capability and biosynthesized acyl-phloroglucinol in addition to wild-type PKSIIINc products. Generation of alkyl-resorcinol and acyl-phloroglucinol by a single protein is a unique observation in fungal type III PKS family. Mutation of Cys120 to bulky Phe side-chain abrogated formation of Tetraketide products and adversely affected overall protein stability as revealed by molecular dynamics simulation studies. Our investigations identify residue positions governing cyclization programming in PKSIIINc protein and provide insights on how subtle variations in protein cores dictate product profiles in type III PKS family.
Molecular characterization of two alkylresorcylic acid synthases from Sordariomycetes fungi.[Pubmed:29859598]
Enzyme Microb Technol. 2018 Aug;115:16-22.
Two putative type III polyketide synthase genes (PKS) were identified from Sordariomycetes fungi. These two type III PKS genes from Sordaria macrospora (SmPKS) and Chaetomium thermophilum (CtPKS), shared 59.8% sequence identity. Both, full-length and truncated versions of type III PKSs were successfully cloned and overexpressed in a bacterial host, Escherichia Coli BL21 (DE3) using a N-terminus hexa-histidine tag. The full-length and the truncated construct of PKSs showed similar activity profiles, suggesting that additional amino acid residues at the C-terminal of both SmPKS and CtPKS may not be involved in catalytic functions. We demonstrate that these two recombinant polyketide synthases could efficiently synthesize tri- and Tetraketide pyrones, resorcinols and resorcylic acids using various acyl-CoAs (C4-C20) as starter units. The truncated S. macrospora polyketide synthases (TrSmPKS) showed a maximum of 7.0x10(4)s(-1)M(-1) catalytic efficiency towards stearoyl-CoA.Whereas, truncated C. thermophilum polyketide synthases (TrCtPKS) preferred the long-chain acyl-CoA starter arachidoyl-CoA, to produce pentaketide and hexaketide resorcinols with a high catalytic efficiency of 6.2x10(4)s(-1)M(-1). Homology model and substrate docking analyses suggest a shorter distance between sulfur of catalytic Cys152 and thioester carbonyl group of arachidoyl-CoA as well as stronger imidazolium-thiolate ion pair distance in TrCtPKS between catalytic Cys152-His309 compared to TrSmPKS- arachidoyl CoA complex. Enhanced binding interactions of CtPKS residues forming intermolecular contacts at the active site could be attributed to its high specificity towards arachidoyl-CoA. This study reports the functional characterization of two fungal type III polyketide synthases, SmPKS and CtPKS with high catalytic efficiency from S. macrospora and C. thermophilum respectively. Furthermore, the results suggested that the both SmPKS and CtPKS could be attractive targets for protein engineering to discern the unique substrate specificity and catalytic efficiency.
A unified mechanism for plant polyketide biosynthesis derived from in silico modeling.[Pubmed:29496450]
Biochem Biophys Res Commun. 2018 Mar 18;497(4):1123-1128.
The polyketide synthases found in a variety of plants and fungi provide a varied source of biologically active compounds of pharmacological and medicinal interest. Stilbene synthase and chalcone synthase catalyze the formation of a common Tetraketide intermediate, but use different cyclization mechanisms to produce distinct and separate natural products. While key structural differences have been identified to explain this functional diversity, a fuller explication of the factors responsible for this mechanistic disparity is required. Based on the energetics of our models of the bound Tetraketides, and our structural analysis of the active sites we propose that a key tautomeric conversion provides a mechanistic framework common to both cyclizations. A previously unidentified active water molecule facilitates cyclization in chalcone synthase through a Claisen mechanism. Such a "Claisen switch" is comparable to the previously characterized "aldol switch" mechanism proposed for the biosynthesis of resveratrol in stilbene synthase.
Structural and biochemical characterization of the plant type III polyketide synthases of the liverwort Marchantia paleacea.[Pubmed:29428820]
Plant Physiol Biochem. 2018 Apr;125:95-105.
Chalcone synthases (CHSs) of the type III polyketide synthases (PKSs), catalyze the formation of a Tetraketide intermediate from a CoA-tethered starter and malonyl-CoA but use different cyclization mechanisms to produce distinct chemical scaffolds. Herein, we characterized CHS and CHS-like enzymes (designated MpCHS and MpCHSL1, 2 and 3) from Marchantia paleacea and determined the crystal structure of MpCHSL1. MpCHS catalyzed a Claisen condensation to form chalcone, while MpCHSLs catalyzed the formation of lactonized alpha-pyrones in vitro. Based on the structural, mutational and in vitro biochemical analyses, we established that MpCHSL1 is structurally and functionally closer to prototype CHS than stilbene synthase, and characterized the structural basis for the functional diversity of the type III PKSs. A chalcone-forming mutant of MpCHSL1 was build directed by the structural information. These findings pave the way for future studies to elucidate the functional diversity of type III PKSs in liverwort.


