TonabersatGap junction modulator,novel benzopyran compound for Migraine and epilepsy treatment CAS# 175013-84-0 |
- Mozavaptan
Catalog No.:BCC5095
CAS No.:137975-06-5
- Desmopressin
Catalog No.:BCC1525
CAS No.:16679-58-6
- Conivaptan HCl
Catalog No.:BCC3756
CAS No.:168626-94-6
- Desmopressin Acetate
Catalog No.:BCC1526
CAS No.:62288-83-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 175013-84-0 | SDF | Download SDF |
| PubChem ID | 3055165 | Appearance | Powder |
| Formula | C20H19ClFNO4 | M.Wt | 391.82 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | SB-220453 | ||
| Solubility | DMSO : ≥ 100 mg/mL (255.22 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
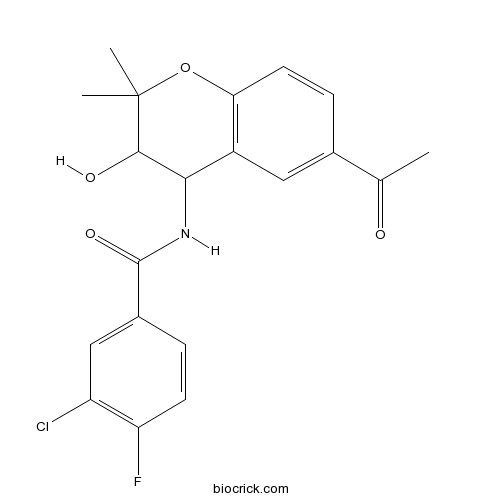
| Chemical Name | N-(6-acetyl-3-hydroxy-2,2-dimethyl-3,4-dihydrochromen-4-yl)-3-chloro-4-fluorobenzamide | ||
| SMILES | CC(=O)C1=CC2=C(C=C1)OC(C(C2NC(=O)C3=CC(=C(C=C3)F)Cl)O)(C)C | ||
| Standard InChIKey | XLIIRNOPGJTBJD-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C20H19ClFNO4/c1-10(24)11-5-7-16-13(8-11)17(18(25)20(2,3)27-16)23-19(26)12-4-6-15(22)14(21)9-12/h4-9,17-18,25H,1-3H3,(H,23,26) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Tonabersat is a modulator of gap-junction with IC50 value of 0.5 µM for the electrographic bursting in the K+ hippocampal brain slice model. | |||||
| Targets | gap-junction | |||||

Tonabersat Dilution Calculator

Tonabersat Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5522 mL | 12.761 mL | 25.5219 mL | 51.0438 mL | 63.8048 mL |
| 5 mM | 0.5104 mL | 2.5522 mL | 5.1044 mL | 10.2088 mL | 12.761 mL |
| 10 mM | 0.2552 mL | 1.2761 mL | 2.5522 mL | 5.1044 mL | 6.3805 mL |
| 50 mM | 0.051 mL | 0.2552 mL | 0.5104 mL | 1.0209 mL | 1.2761 mL |
| 100 mM | 0.0255 mL | 0.1276 mL | 0.2552 mL | 0.5104 mL | 0.638 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Migraine is a common, recurrent and primary headache disorder. Optimisation of novel cis- and trans-4-(substituted-anfido)benzopyran-3-ol derivatives has led to the identification of Tonabersat (SB-220453) with potential antimigraine activity.
In vitro: Topiramate targets multiple cortical and subcortical loci, altering voltage-gated ion channels and chemical transmission to decrease abnormal brain excitability. Preclinical studies have identified four properties that may account for the drug’s efficacy in epilepsy and migraine prophylaxis: (i) blockage of voltage-dependent sodium channels; (ii) augmentation of the activity of the neurotransmitter g-aminobutyric acid (GABA) at some subtypes of the GABA-A receptor; (iii) antagonism of the AMPA/kainate subtype of the glutamate receptor; and (iv) inhibition of the carbonic anhydrase enzymes, particularly isozymes II and IV [1].
In vivo: Tonabersat binds selectively to a unique site in the brain. Moreover, tonabersat could markedly reduce cortical spreading depression (CSD) and CSD-associated events and inhibite gap-junction communication between neurons and satellite glial cells in the trigeminal ganglion. Together, these findings indicate that tonabersat should have clinical application in preventing migraine attacks [2].
Clinical trial: Tonabersat showed a preventive eff ect on attacks of migraine aura but no efficacy on non-aura attacks, in keeping with its known inhibitory eff ect on CSD. The results support the theory that auras are caused by CSD and that this phenomenon is not involved in attacks without aura [3].
References:
[1] Silberstein SD. Tonabersat, a novel gap-junction modulator for the prevention of migraine. Cephalalgia. 2009;29 Suppl 2:28-35.
[2] Durham PL, Garrett FG. Neurological mechanisms of migraine: potential of the gap-junction modulator tonabersat in prevention of migraine. Cephalalgia. 2009;29 Suppl 2:1-6.
[3] Hauge AW, Asghar MS, Schytz HW, Christensen K, Olesen J. Effects of tonabersat on migraine with aura: a randomised, double-blind, placebo-controlled crossover study. Lancet Neurol. 2009;8(8):718-23.
- Parishin C
Catalog No.:BCN3813
CAS No.:174972-80-6
- Parishin B
Catalog No.:BCN3812
CAS No.:174972-79-3
- Rabdoketone B
Catalog No.:BCN6598
CAS No.:174819-51-3
- 3-Amino-4-methoxybenzamide
Catalog No.:BCC8612
CAS No.:17481-27-5
- Fmoc-Hyp(Bzl)-OH
Catalog No.:BCC3255
CAS No.:174800-02-3
- Carabrone
Catalog No.:BCN1121
CAS No.:1748-81-8
- Ginsenoside Rh4
Catalog No.:BCN3503
CAS No.:174721-08-5
- 2-Amino-6-methoxybenzothiazole
Catalog No.:BCC8542
CAS No.:1747-60-0
- CH 275
Catalog No.:BCC5913
CAS No.:174688-78-9
- AN-2690
Catalog No.:BCC1360
CAS No.:174671-46-6
- Phalloidin
Catalog No.:BCC7945
CAS No.:17466-45-4
- Talnetant
Catalog No.:BCC1981
CAS No.:174636-32-9
- NQDI 1
Catalog No.:BCC2404
CAS No.:175026-96-7
- O4I1
Catalog No.:BCC6542
CAS No.:175135-47-4
- Xanthorin
Catalog No.:BCN1122
CAS No.:17526-15-7
- Fmoc-Thr(HPO3Bzl)-OH
Catalog No.:BCC3551
CAS No.:175291-56-2
- S 18986
Catalog No.:BCC6081
CAS No.:175340-20-2
- Voreloxin
Catalog No.:BCC2044
CAS No.:175414-77-4
- Z-Phe(4-F)-OH
Catalog No.:BCC3221
CAS No.:17543-58-7
- Eriosematin A
Catalog No.:BCN3465
CAS No.:175448-02-9
- Fmoc-3-Pal-OH
Catalog No.:BCC2653
CAS No.:175453-07-3
- Fmoc-Phe(4-Cl)-OH
Catalog No.:BCC3173
CAS No.:175453-08-4
- Voreloxin Hydrochloride
Catalog No.:BCC2045
CAS No.:175519-16-1
- Longifloroside A
Catalog No.:BCN1123
CAS No.:175556-08-8
Tonabersat for migraine prophylaxis: a systematic review.[Pubmed:24452641]
Pain Physician. 2014 Jan-Feb;17(1):1-8.
BACKGROUND: Randomized clinical trials assessing the efficacy and tolerability of Tonabersat compared with placebo as prophylaxis for migraine were systematically reviewed in this study. By analyzing all available data, we aimed to establish an overall estimate of any association in order to more accurately inform clinicians and care-givers about how to prevent migraines. OBJECTIVE: To evaluate the efficacy and tolerability of Tonabersat when it is used for migraine prevention. STUDY DESIGN: Systematic review of Tonabersat for migraine prophylaxis. METHODS: Computerized database search of The Cochrane Pain, Palliative & Supportive Care Trials Register, The Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library), Pubmed, and EMBASE for randomized, double-blind, placebo-controlled trials on Tonabersat for migraine until January, 2013. We also searched the ongoing trials. We did not impose any language restrictions.The quality assessment and clinical relevance criteria utilized were the Cochrane Pain, Palliative & Supportive Care review group criteria as utilized for randomized trials. OUTCOME MEASURES: The primary outcome measure was the change in mean number of migraine headache days. The secondary outcome measures were change in attacks, responder rates, the reduction of the consumption of rescue medication, and adverse events. RESULTS: For this systematic review, 133 studies were identified. Of these, 131 studies were excluded, and a total of 2 studies (after removal of duplicate publications) met inclusion criteria for methodological quality assessment with the randomized trial study. The evidence for Tonabersat for migration prophylaxis failed to demonstrate a reduction when compared to placebo because of a lack of evidence. But the good tolerability supports further exploration of Tonabersat in the prevention of migraine attacks. LIMITATIONS: The limitation of this systematic review was a lack of available evidence. CONCLUSION: There is fair evidence for migraine prophylaxis, but a lack of available evidence for Tonabersat for migraine prophylaxis. Although Tonabersat failed to demonstrate a significantly greater reduction of migraine headache days than placebo, it was well tolerated. Future work should further investigate the utility of Tonabersat in the preventive management of migraine.
Tonabersat, a gap-junction modulator: efficacy and safety in two randomized, placebo-controlled, dose-ranging studies of acute migraine.[Pubmed:19723122]
Cephalalgia. 2009 Nov;29 Suppl 2:17-27.
Tonabersat is a novel benzopyran derivative that blocks the cortical spreading depression proposed to be associated with migraine attacks. The ability of single oral doses of 15, 25, 40 and 80 mg of Tonabersat to relieve the symptoms of moderate to severe migraine was evaluated in 859 migraineurs enrolled in two dose-ranging, double-blind, randomized, placebo-controlled, parallel-group trials, one international and the other North American. In the international study, significantly more patients given Tonabersat than given placebo experienced relief of headache pain at 2 h (15 mg, 36.8%; 40 mg, 40.7%), the principal efficacy variable, and at 4 h (40 mg, 63.0%) and complete abolition of headache at 4 h (40 mg, 34.3%). None of the primary or secondary efficacy variables indicated significant differences between Tonabersat and placebo in the North American study. Tonabersat was generally well tolerated, with dizziness and nausea the most common side-effects. Serious adverse events were uncommon, and no patient withdrew from either study because of adverse events. These results suggest a possible interplay between Tonabersat pharmacokinetics (the relatively long time required to reach maximum plasma concentrations) and patient characteristics (previous triptan exposure) in the management of acute migraine attacks. Based on the pharmacokinetics and actions on cortical spreading depression, Tonabersat may have potential value in migraine prophylaxis.
Tonabersat, a novel gap-junction modulator for the prevention of migraine.[Pubmed:19723123]
Cephalalgia. 2009 Nov;29 Suppl 2:28-35.
Migraine is a common, recurrent, primary headache disorder associated with significant morbidity as well as high direct and indirect costs. Despite its impact, only a proportion of migraineurs who meet criteria for prophylactic treatment take preventive medication. Antiepileptic drugs and beta-blockers are among the most used preventive therapies, but their exact mechanisms of action in migraine prophylaxis are unknown. Recent research has pointed to the role of cortical spreading depression in the genesis of migraine aura and pain, with neuronal-glial gap junctions playing a prominent part in cortical spreading depression. Tonabersat is a unique compound with demonstrated activity as a gap-junction inhibitor in animal studies. In preclinical and clinical trials, Tonabersat was well tolerated, with no cardiovascular effects; the pharmacokinetic profile suggested its usefulness in the prophylaxis of migraine.


