Turmeronol BCAS# 131651-38-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 131651-38-2 | SDF | Download SDF |
| PubChem ID | 11770338 | Appearance | Oil |
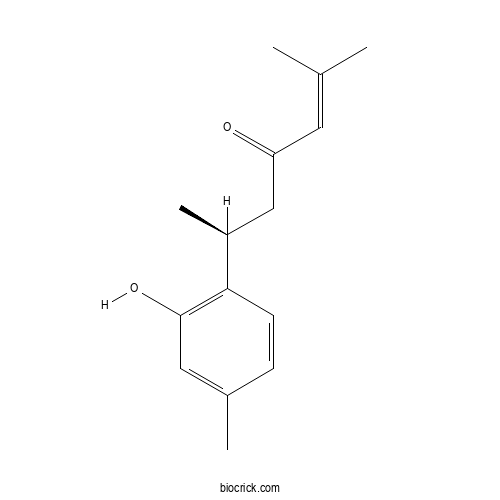
| Formula | C15H20O2 | M.Wt | 232.32 |
| Type of Compound | Sesquiterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (6S)-6-(2-hydroxy-4-methylphenyl)-2-methylhept-2-en-4-one | ||
| SMILES | CC1=CC(=C(C=C1)C(C)CC(=O)C=C(C)C)O | ||
| Standard InChIKey | WYIJOOQDLOBLCP-LBPRGKRZSA-N | ||
| Standard InChI | InChI=1S/C15H20O2/c1-10(2)7-13(16)9-12(4)14-6-5-11(3)8-15(14)17/h5-8,12,17H,9H2,1-4H3/t12-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Turmeronol B Dilution Calculator

Turmeronol B Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.3044 mL | 21.522 mL | 43.0441 mL | 86.0882 mL | 107.6102 mL |
| 5 mM | 0.8609 mL | 4.3044 mL | 8.6088 mL | 17.2176 mL | 21.522 mL |
| 10 mM | 0.4304 mL | 2.1522 mL | 4.3044 mL | 8.6088 mL | 10.761 mL |
| 50 mM | 0.0861 mL | 0.4304 mL | 0.8609 mL | 1.7218 mL | 2.1522 mL |
| 100 mM | 0.043 mL | 0.2152 mL | 0.4304 mL | 0.8609 mL | 1.0761 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Tinospin E
Catalog No.:BCX0233
CAS No.:1582321-96-7
- N-Acetylnornuciferine
Catalog No.:BCX0232
CAS No.:1942-03-6
- Fibleucin
Catalog No.:BCX0231
CAS No.:24278-14-6
- Apigenin 6-C-(2-O-feruloyl)glucoside 8-C-glucoside
Catalog No.:BCX0230
CAS No.:1287786-63-3
- Makisterone A
Catalog No.:BCX0229
CAS No.:20137-14-8
- Brasilixanthone B
Catalog No.:BCX0228
CAS No.:84002-57-3
- 22-Epiinotodiol
Catalog No.:BCX0227
CAS No.:64907-49-9
- 1,3,5,7-Tetrahydroxy-8-prenylxanthone
Catalog No.:BCX0226
CAS No.:444004-76-6
- 1,6,8-Trihydroxy-2,7-dimethoxy-3-methylanthraquinone
Catalog No.:BCX0225
CAS No.:2366153-27-5
- Phenacetamide
Catalog No.:BCX0224
CAS No.:103-81-1
- 4-(1-Ethoxy-2-hydroxyethyl)benzene-1,2-diol
Catalog No.:BCX0223
CAS No.:1190632-33-7
- 3β,5α-Dihydroxystigmastan-6-one
Catalog No.:BCX0222
CAS No.:55051-78-0
- Aristolodione
Catalog No.:BCX0235
CAS No.:109771-09-7
- Ciwujianoside C2
Catalog No.:BCX0236
CAS No.:114892-56-7
- Mckeanianone B
Catalog No.:BCX0237
CAS No.:2035475-14-8
- 1-O-β-D-Glucopyranosylpaeonisuffrone
Catalog No.:BCX0238
CAS No.:1003888-20-7
- Pteroside B
Catalog No.:BCX0239
CAS No.:29774-74-1
- Kaempferol 3-O-neohesperidoside 7-O-glucoside
Catalog No.:BCX0240
CAS No.:78527-48-7
- Creticoside A
Catalog No.:BCX0241
CAS No.:34336-00-0
- (S,E)-2-Methyl-6-(p-tolyl)hept-3-en-2-ol
Catalog No.:BCX0242
CAS No.:18383-55-6
- ar-Turmerol
Catalog No.:BCX0243
CAS No.:1178899-16-5
- Bisacurone A
Catalog No.:BCX0244
CAS No.:127214-84-0
- Malaysianol D
Catalog No.:BCX0245
CAS No.:1646330-59-7
- Pyridylpaeoniflorin
Catalog No.:BCX0246
CAS No.:1427054-19-0
Curcuma longa extract reduces serum inflammatory markers and postprandial hyperglycemia in healthy but borderline participants with overweight and glycemia in the normal/prediabetes range: a randomized, double-blind, and placebo-controlled trial.[Pubmed:38347961]
Front Nutr. 2024 Jan 29;11:1324196.
The spice turmeric, which has the Latin name Curcuma longa (C. longa), has various physiological effects. This study evaluated the effects of a hot water mixture with supercritical carbon dioxide C. longa extracts, CLE, and the potential active components of C. longa, turmeronols A and B and bisacurone on inflammation and glucose metabolism. First, we investigated the effect of CLE and the potential active components of C. longa on lipopolysaccharide-induced inflammation in RAW264.7 macrophages. We found a significant decrease in the production of interleukin (IL)-1beta, IL-6, tumor necrosis factor (TNF)-alpha, and nitric oxide with CLE, turmeronol A, and bisacurone, Significant inhibition of each of these substances was also observed, except for TNF-alpha with Turmeronol B. The second part of our work was a 12-week randomized, double-blind, placebo-controlled study in healthy but borderline adults aged 40 to 69 years with overweight and normal/prediabetes glycemia. We compared blood inflammatory and glycometabolic markers in the CLE (n = 55) and placebo groups (n = 55). We found significantly lower serum high-sensitivity C-reactive protein and hemoglobin A1c levels in the CLE group. This group also showed significant improvements in postprandial hyperglycemia and insulin sensitivity indices. Our findings indicate that CLE may reduce low-grade inflammation and thus improve insulin sensitivity and postprandial hyperglycemia. Clinical trial registration: https://center6.umin.ac.jp/cgi-open-bin/ctr/ctr_view.cgi?recptno=R000051492, UMIN-CTR, UMIN000045106.
Correction: Turmeronol A and turmeronol B from Curcuma longa prevent inflammatory mediator production by lipopolysaccharide-stimulated RAW264.7 macrophages, partially via reduced NF-kappaB signaling.[Pubmed:33135696]
Food Funct. 2020 Nov 18;11(11):10243.
Correction for 'Turmeronol A and Turmeronol B from Curcuma longa prevent inflammatory mediator production by lipopolysaccharide-stimulated RAW264.7 macrophages, partially via reduced NF-kappaB signaling' by Chinatsu Okuda-Hanafusa et al., Food Funct., 2019, 10, 5779-5788. DOI: 10.1039/C9FO00336C.
Turmeronol A and turmeronol B from Curcuma longa prevent inflammatory mediator production by lipopolysaccharide-stimulated RAW264.7 macrophages, partially via reduced NF-kappaB signaling.[Pubmed:31454011]
Food Funct. 2019 Sep 1;10(9):5779-5788.
Chronic inflammation depends on inflammatory mediators produced by activated macrophages and is the common pathological basis for various diseases. Turmeronol is a sesquiterpenoid found in the spice turmeric (Curcuma longa), which is known to have anti-inflammatory activity. To elucidate the anti-inflammatory mechanism of turmeronol, we investigated the influence of turmeronol A and Turmeronol B in mouse macrophages (RAW264.7 cells) stimulated with lipopolysaccharide (LPS). Pretreatment of RAW264.7 cells with either turmeronol A or B significantly inhibited LPS-induced production of prostaglandin E(2) and nitric oxide, as well as expression of mRNAs for the corresponding synthetic enzymes. In addition, the turmeronols significantly inhibited LPS-induced upregulation of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha at the mRNA and protein levels. Both turmeronols also inhibited nuclear translocation of nuclear factor kappaB (NF-kappaB), with a similar time course to the NF-kappaB inhibitor pyrrolidine dithiocarbamate, but not curcumin (another NF-kappaB inhibitor). Thus, both turmeronols prevented activation of macrophages and inflammatory mediator production, possibly by suppressing activation of NF-kappaB, and therefore have potential for use in preventing chronic inflammatory diseases.
Hot Water Extract of Curcuma longa L. Improves Serum Inflammatory Markers and General Health in Subjects with Overweight or Prehypertension/Mild Hypertension: A Randomized, Double-Blind, Placebo-Controlled Trial.[Pubmed:31394768]
Nutrients. 2019 Aug 7;11(8):1822.
To investigate the effect of a hot water extract of C. longa L. (WEC) containing anti-inflammatory agents, bisacurone, and turmeronol on chronic inflammation, a randomized double-blind placebo-controlled study was conducted in middle-aged and elderly subjects aged 50-69 years with overweight or prehypertension/mild hypertension. The subjects consumed 900 mg WEC tablets, containing 400 mug bisacurone, 80 mug turmeronol A and 20 mug Turmeronol B (WEC group: n = 45), or placebo tablets without WEC (placebo group: n = 45) daily for 12 weeks. Serum inflammatory and metabolic markers were measured. The subjects also completed the MOS 36-item short-form health survey (SF-36) and the Profile of Mood States scale (POMS). In the WEC group, the serum levels of C-reactive protein, tumor necrosis factor-alpha, interleukin-6, and soluble vascular cell adhesion molecule-1 decreased significantly. Compared with the placebo group, the WEC group had significantly lower serum levels of glucose, hemoglobin A1c, and triglycerides, as well as higher serum levels of high-density lipoprotein cholesterol. The WEC group also showed significant improvement of SF-36 scores (for general health, vitality, mental health, and mental summary component) and POMS scores for positive mood states (vigor-activity and friendliness). In conclusion, WEC may ameliorate chronic low-grade inflammation, thus contributing to the improvement of associated metabolic disorders and general health.
Isolation and structural elucidation of antifungal compounds from Ryudai gold (Curcuma longa) against Fusarium solani sensu lato isolated from American manatee.[Pubmed:30802619]
Comp Biochem Physiol C Toxicol Pharmacol. 2019 May;219:87-94.
In a previous study, we reported that Curcuma longa strain Ryudai gold (RD) showed antifungal activity against Fusarium solani sensu lato (FSSL) among the different species and varieties of turmeric. The present study focused on isolation, identification and structural elucidation of antifungal compounds in RD. The ethyl acetate (EtOAc) fraction was eluted with n-hexane and EtOAc with gradually increasing the concentration of EtOAc (n-hexane:EtOAc; 100:0; 80:20; 60:40, 40:60, 20:80 and 0:100). The antifungal compounds were isolated from the most effective fraction by using silica gel, TOYOPEARL(R) HW-40F column, and high-performance liquid chromatography. Structural identification of the antifungal compounds was conducted using (1)H NMR, (13)C NMR, and liquid chromatography-tandem mass spectrometry. The MeOH extract of the rhizome of RD inhibited the growth of FSSL in a concentration-dependent manner. The EtOAc fraction of the MeOH extract of RD demonstrated the highest antifungal activity against FSSL. The purified antifungal compounds were Turmeronol B (1), turmeronol A (2), (E)-alpha-atlantone (3), dihydrobisdemethoxycurcumin (4), demethoxycurcumin (5) and curcumin (6). These six compounds showed concentration-dependent antifungal activity against FSSL. The concentration required for 50% growth inhibition (IC(50)) of the four isolates of FSSL ranged from 116 to172, 127 to 185, 88 to 109, 90 to 112, 74 to 80 and 63 to 68 muM/L for Turmeronol B, turmeronol A, (E)-alpha-atlantone, dihydrobisdemethoxycurcumin, demethoxycurcumin and curcumin, respectively. These results suggested that RD contained potential antifungal compounds that could be useful to control FSSL. The isolated compounds of RD can be a good source of natural antifungal agents or the lead compounds for the development of new synthetic drugs.
Antioxidant activity of different species and varieties of turmeric (Curcuma spp): Isolation of active compounds.[Pubmed:30266519]
Comp Biochem Physiol C Toxicol Pharmacol. 2019 Jan;215:9-17.
There are >80 species of turmeric (Curcuma spp.) and some species have multiple varieties, for example, Curcuma longa (C. longa) has 70 varieties. They could be different in their chemical properties and biological activities. Therefore, we compared antioxidant activity, total phenolic and flavonoid content of different species and varieties of turmeric namely C. longa [variety: Ryudai gold (RD) and Okinawa ukon], C. xanthorrhiza, C. aromatica, C. amada, and C. zedoaria. The antioxidant activity was determined using the 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical scavenging activity, oxygen radical absorbance capacity (ORAC), reducing power and 2-deoxyribose (2-DR) oxidation assay. Our results suggested that RD contained significantly higher concentrations of total phenolic (157.4 mg gallic acid equivalent/g extract) and flavonoids (1089.5 mg rutin equivalent/g extract). RD also showed significantly higher DPPH radical-scavenging activity (IC(50): 26.4 mug/mL), ORAC (14,090 mumol Trolox equivalent/g extract), reducing power absorbance (0.33) and hydroxyl radical scavenging activity (IC(50): 7.4 mug/mL). Therefore, RD was chosen for the isolation of antioxidant compounds using silica gel column, Toyopearl HW-40F column, and high-performance liquid chromatography. Structural identification of the compounds was conducted using (1)H NMR, (13)C NMR, and liquid chromatography-tandem mass spectrometry. The purified antioxidant compounds were bisabolone-9-one (1), 4-methyllene-5-hydroxybisabola-2,10-diene-9-one (2), Turmeronol B (3), 5-hydroxy-1,7-bis(4-hydroxy-3-methoxyphenyl)-1-hepten-3-one (4), 3-hydroxy-1,7-bis(4-hydroxyphenyl)-6-hepten-1,5-dione (5), cyclobisdemethoxycurcumin (6), bisdemethoxycurcumin (7), demethoxycurcumin (8) and curcumin (9). The IC(50) for DPPH radical-scavenging activity were 474, 621, 234, 29, 39, 257, 198, 47 and 18 muM and hydroxyl radical-scavenging activity were 25.1, 24.4, 20.2, 2.1, 5.1, 17.2, 7.2, 3.3 and 1.5 muM for compound 1, 2, 3, 4, 5, 6, 7, 8 and 9, respectively. Our findings suggested that the RD variety of C. longa, developed by the University of the Ryukyus, Okinawa, Japan, is a promising source of natural antioxidants.


