Malaysianol DCAS# 1646330-59-7 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 1646330-59-7 | SDF | File under preparation. |
| PubChem ID | N/A | Appearance | Powder |
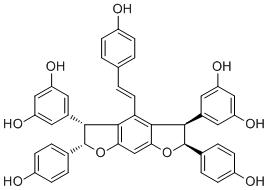
| Formula | C42H32O9 | M.Wt | 680.74 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Malaysianol D Dilution Calculator

Malaysianol D Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.469 mL | 7.3449 mL | 14.6899 mL | 29.3798 mL | 36.7247 mL |
| 5 mM | 0.2938 mL | 1.469 mL | 2.938 mL | 5.876 mL | 7.3449 mL |
| 10 mM | 0.1469 mL | 0.7345 mL | 1.469 mL | 2.938 mL | 3.6725 mL |
| 50 mM | 0.0294 mL | 0.1469 mL | 0.2938 mL | 0.5876 mL | 0.7345 mL |
| 100 mM | 0.0147 mL | 0.0734 mL | 0.1469 mL | 0.2938 mL | 0.3672 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Bisacurone A
Catalog No.:BCX0244
CAS No.:127214-84-0
- ar-Turmerol
Catalog No.:BCX0243
CAS No.:1178899-16-5
- (S,E)-2-Methyl-6-(p-tolyl)hept-3-en-2-ol
Catalog No.:BCX0242
CAS No.:18383-55-6
- Creticoside A
Catalog No.:BCX0241
CAS No.:34336-00-0
- Kaempferol 3-O-neohesperidoside 7-O-glucoside
Catalog No.:BCX0240
CAS No.:78527-48-7
- Pteroside B
Catalog No.:BCX0239
CAS No.:29774-74-1
- 1-O-β-D-Glucopyranosylpaeonisuffrone
Catalog No.:BCX0238
CAS No.:1003888-20-7
- Mckeanianone B
Catalog No.:BCX0237
CAS No.:2035475-14-8
- Ciwujianoside C2
Catalog No.:BCX0236
CAS No.:114892-56-7
- Aristolodione
Catalog No.:BCX0235
CAS No.:109771-09-7
- Turmeronol B
Catalog No.:BCX0234
CAS No.:131651-38-2
- Tinospin E
Catalog No.:BCX0233
CAS No.:1582321-96-7
- Pyridylpaeoniflorin
Catalog No.:BCX0246
CAS No.:1427054-19-0
- Pterosin L 2'-O-glucoside
Catalog No.:BCX0247
CAS No.:61102-11-2
- (2R,3S)-Pteroside C
Catalog No.:BCX0248
CAS No.:68399-16-6
- (2S,3S)-Pteroside C
Catalog No.:BCX0249
CAS No.:98855-62-0
- Dehydrozingerone
Catalog No.:BCX0250
CAS No.:1080-12-2
- Kaempferol 3-O-(2''-O-glucosyl)rutinoside
Catalog No.:BCX0251
CAS No.:55696-58-7
- Sanggenon B
Catalog No.:BCX0252
CAS No.:81381-67-1
- Chasmanthin
Catalog No.:BCX0253
CAS No.:20379-19-5
- Dulcisxanthone B
Catalog No.:BCX0254
CAS No.:869669-62-5
- (E)-4-(4-Hydroxyphenyl)but-3-en-2-one
Catalog No.:BCX0255
CAS No.:22214-30-8
- (E)-α-Atlantone
Catalog No.:BCX0256
CAS No.:26294-59-7
- Arucadiol
Catalog No.:BCX0257
CAS No.:105037-85-2
Induction of apoptosis and G2/M arrest by ampelopsin E from Dryobalanops towards triple negative breast cancer cells, MDA-MB-231.[Pubmed:27609190]
BMC Complement Altern Med. 2016 Sep 8;16(1):354.
BACKGROUND: Several compounds isolated from Dryobalanops have been reported to exhibit cytotoxic effects to several cancer cell lines. This study investigated the cytotoxic effects, cell cycle arrest and mode of cell death in ampelopsin E-treated triple negative cells, MDA-MB-231. METHODS: Cytotoxicity of ampelopsin E, ampelopsin F, flexuosol A, laevifonol, Malaysianol A, Malaysianol D and nepalensinol E isolated from Dryobalanops towards human colon cancer HT-29, breast cancer MDA-MB-231 and MCF-7, alveolar carcinoma HeLa and mouse embryonic fibroblast NIH/3 T3 cells were determined by MTT assay. The cells were treated with the compounds (0.94-30 muM) for 72 h. The mode of cell death was evaluated by using an inverted light microscope and annexin V/PI analysis. Cell cycle analysis was performed by using a flow cytometer. RESULTS: Data showed that ampelopsin E was most cytotoxic toward MDA-MB-231 with the IC50 (50 % inhibition of cell viability compared to control) of 14.5 +/- 0.71 muM at 72 h. Cell shrinkage, membrane blebbing and formation apoptotic bodies characteristic of apoptosis were observed following treatment with ampelopsin E. The annexin V/PI flow cytometric analysis further confirmed that ampelopsin E induced apoptosis in MDA-MB-231 cells. Cell cycle analysis revealed that ampelopsin E induced G2/M phase cell cycle arrest in the cells. CONCLUSION: Ampelopsin E induced apoptosis and cell cycle arrest in MDA-MB-231 cells. Therefore, ampelopsin E has the potential to be developed into an anticancer agent for treatment of triple negative breast cancer.


