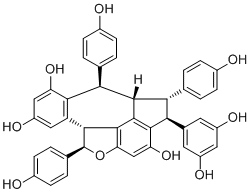
Vaticanol ACAS# 287101-82-0 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 287101-82-0 | SDF | Download SDF |
| PubChem ID | N/A | Appearance | Brown powder |
| Formula | C42H32O9 | M.Wt | 680.74 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Vaticanol A Dilution Calculator

Vaticanol A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.469 mL | 7.3449 mL | 14.6899 mL | 29.3798 mL | 36.7247 mL |
| 5 mM | 0.2938 mL | 1.469 mL | 2.938 mL | 5.876 mL | 7.3449 mL |
| 10 mM | 0.1469 mL | 0.7345 mL | 1.469 mL | 2.938 mL | 3.6725 mL |
| 50 mM | 0.0294 mL | 0.1469 mL | 0.2938 mL | 0.5876 mL | 0.7345 mL |
| 100 mM | 0.0147 mL | 0.0734 mL | 0.1469 mL | 0.2938 mL | 0.3672 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Zedoalactone E
Catalog No.:BCX0332
CAS No.:1228391-67-0
- Castanol A
Catalog No.:BCX0331
CAS No.:1437630-15-3
- (1R,16R)-1-Hydroxycryptotanshinone anhydride
Catalog No.:BCX0330
CAS No.:2132963-89-2
- 2'-O-(2,3-Dihydroxybenzoyl)sweroside
Catalog No.:BCX0329
CAS No.:172518-55-7
- Curdionolide A
Catalog No.:BCX0328
CAS No.:1190225-67-2
- Zedoalactone C
Catalog No.:BCX0327
CAS No.:864430-46-6
- Trijugin F
Catalog No.:BCX0326
CAS No.:1233534-51-4
- Macrophylloside B
Catalog No.:BCX0325
CAS No.:179457-67-1
- Trijugin D
Catalog No.:BCX0324
CAS No.:1233534-47-8
- Macrophylloside A
Catalog No.:BCX0323
CAS No.:179457-66-0
- Peruvianoside I
Catalog No.:BCX0322
CAS No.:450407-24-6
- Secoaristolenedioic acid
Catalog No.:BCX0321
CAS No.:2756011-83-1
- α-Amyrin caffeate
Catalog No.:BCX0334
CAS No.:1360077-48-0
- Hydroxydihydrobovolide
Catalog No.:BCX0335
CAS No.:1403940-81-7
- Conicaoside
Catalog No.:BCX0336
CAS No.:916888-05-6
- β-Amyrin caffeate
Catalog No.:BCX0337
CAS No.:485842-75-9
- Phaeocaulisin I
Catalog No.:BCX0338
CAS No.:1438899-53-6
- Anisolactone
Catalog No.:BCX0339
CAS No.:94418-50-5
- Lucidafuranocoumarin B
Catalog No.:BCX0340
CAS No.:1422520-77-1
- 12β-Hydroxyprogesterone
Catalog No.:BCX0341
CAS No.:29332-92-1
- Yunnancoronarin B
Catalog No.:BCX0342
CAS No.:162762-94-9
- Eriocalyxin A
Catalog No.:BCX0343
CAS No.:84745-93-7
- 8-Oxoisocorypalmine
Catalog No.:BCX0344
CAS No.:142808-34-2
- 20(S)-Hydroxypregn-4-en-3-one
Catalog No.:BCX0345
CAS No.:145-14-2
Harnessing quinone methides: total synthesis of (+/-)-vaticanol A.[Pubmed:24841889]
Angew Chem Int Ed Engl. 2014 Jun 23;53(26):6747-51.
Although quinone methides are often postulated as intermediates in the biosynthesis of many polyphenolic natural products, deploying their power in a laboratory setting to achieve similar bond constructions has sometimes proven challenging. Herein, a total synthesis of the resveratrol trimer Vaticanol A has been achieved through three instances of quinone methide chemistry. These operations, one of which succeeded only under very specific conditions, expediently generated its [7,5]-carbocyclic core, afforded a unique sequence for dihydrobenzofuran formation, and concurrently generated, in addition to the target molecule, a series of diastereomers reflective of many other isolates.
Bioactive oligostilbenoids from Shorea maxwelliana King and their chemotaxonomic significance.[Pubmed:23035830]
Nat Prod Res. 2013;27(17):1589-93.
Phytochemical investigation on the stem bark of Shorea maxwelliana yielded five oligostilbenoids identified as alpha-viniferin (1), maximol A (2), Vaticanol A (3), suffruticosol A (4) and vaticanol G (5). Chemotaxonomy of isolated compounds was discussed briefly. Major compounds were tested for neurotoxic and cytotoxic activities. Neurotoxicity for all tested compounds did not pose any toxic effect against cultured cell (cell viability range +/-100-94%). Compounds 2-5 possessed active cyctotoxic activity against HL60 cell line with IC50 values range of 2.7-78 microg mL(-1).
Bioactive oligostilbenoids from the stem bark of Hopea exalata.[Pubmed:17190464]
J Nat Prod. 2006 Dec;69(12):1800-2.
Hopeanolin (1), an unusual resveratral trimer with an ortho-quinone nucleus, was isolated and characterized from the stem bark of Hopea exalata. Also obtained were six known stibenoids, shoreaphenol (2), vaticanol G (3), alpha-viniferin (4), pauciflorol A (5), Vaticanol A (6), and trans-3,5,4'-trihydroxystilbene 2-C-glucoside (7). The structure of 1 was determined by spectroscopic data interpretation. Compounds 1-7 were tested for antifungal activity and inhibitory effects against jack bean urease. Hopeanolin (1) demonstrated antifungal activity in the MIC value range 0.1-22.5 microg/mL.
Anti-babesial activity of some central kalimantan plant extracts and active oligostilbenoids from Shorea balangeran.[Pubmed:15931579]
Planta Med. 2005 May;71(5):420-3.
Bark extracts from a total of 22 species of Central Kalimantan plants were evaluated for their anti-babesial activity against Babesia gibsoni in vitro. Of these plant species, extracts of Calophyllum tetrapterum, Garcinia rigida, Lithocarpus sp., Sandoricum emarginatum, and Shorea balangeran showed more than 90% inhibition of the parasite growth at a test concentration of 1000 microg/mL. Activity-guided fractionation of the bark of S. balangeran (Dipterocarpaceae) led to the reisolation of oligostilbenoids, Vaticanol A(1), B(2), and G(3). The structures were determined on the basis of spectral evidence. Compounds 1 and 3 showed complete inhibition on the growth of Babesia gibsoni in vitro at a concentration of 25 microg/mL, and compound 2 at concentration of 50 microg/mL.


