YM 976PDE4 inhibitor CAS# 191219-80-4 |
- YM155
Catalog No.:BCC2251
CAS No.:781661-94-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 191219-80-4 | SDF | Download SDF |
| PubChem ID | 6604918 | Appearance | Powder |
| Formula | C17H16ClN3O | M.Wt | 313.79 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in ethanol and to 100 mM in DMSO | ||
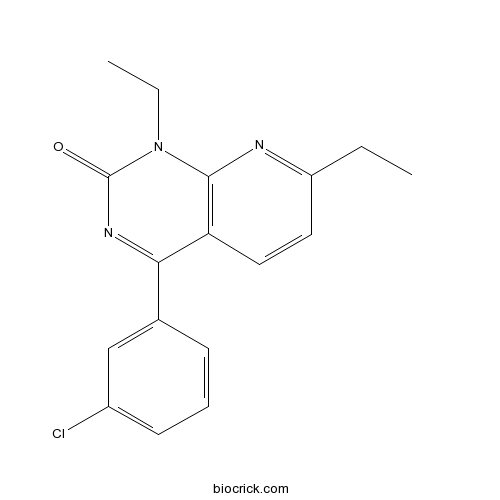
| Chemical Name | 4-(3-chlorophenyl)-1,7-diethylpyrido[2,3-d]pyrimidin-2-one | ||
| SMILES | CCC1=NC2=C(C=C1)C(=NC(=O)N2CC)C3=CC(=CC=C3)Cl | ||
| Standard InChIKey | MNHXYNNKDDXKNP-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C17H16ClN3O/c1-3-13-8-9-14-15(11-6-5-7-12(18)10-11)20-17(22)21(4-2)16(14)19-13/h5-10H,3-4H2,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Orally active PDE4 inhibitor (IC50 = 2.2 nM). Low emetogenic activity, suggested to be due to poor brain penetration. |

YM 976 Dilution Calculator

YM 976 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.1868 mL | 15.9342 mL | 31.8684 mL | 63.7369 mL | 79.6711 mL |
| 5 mM | 0.6374 mL | 3.1868 mL | 6.3737 mL | 12.7474 mL | 15.9342 mL |
| 10 mM | 0.3187 mL | 1.5934 mL | 3.1868 mL | 6.3737 mL | 7.9671 mL |
| 50 mM | 0.0637 mL | 0.3187 mL | 0.6374 mL | 1.2747 mL | 1.5934 mL |
| 100 mM | 0.0319 mL | 0.1593 mL | 0.3187 mL | 0.6374 mL | 0.7967 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Pramipexole 2HCl Monohydrate
Catalog No.:BCC4466
CAS No.:191217-81-9
- Solasonine
Catalog No.:BCN2302
CAS No.:19121-58-5
- Atrazine
Catalog No.:BCC8838
CAS No.:1912-24-9
- Telithromycin
Catalog No.:BCC5273
CAS No.:191114-48-4
- Oplopanone
Catalog No.:BCN1179
CAS No.:1911-78-0
- Kuguacin R
Catalog No.:BCN3057
CAS No.:191097-54-8
- K-7174
Catalog No.:BCC6435
CAS No.:191089-60-8
- L-168,049
Catalog No.:BCC7325
CAS No.:191034-25-0
- Salvigenin
Catalog No.:BCN1178
CAS No.:19103-54-9
- Benzyl 4-Oxo-1-piperidinecarboxylate
Catalog No.:BCC8870
CAS No.:19099-93-5
- Calystegine C2
Catalog No.:BCN1878
CAS No.:190957-44-9
- Triptocallic acid A
Catalog No.:BCN1176
CAS No.:190906-61-7
- C 75
Catalog No.:BCC2386
CAS No.:191282-48-1
- Boc-Asp(OtBu)-OH.DCHA
Catalog No.:BCC3369
CAS No.:1913-12-8
- 1-Deoxynojirimycin
Catalog No.:BCN1032
CAS No.:19130-96-2
- 6-Deoxy-3-O-methyl-beta-allopyranosyl(1-4)-beta-cymaronic acid delta-lactone
Catalog No.:BCN1514
CAS No.:19131-13-6
- Ursolic aldehyde
Catalog No.:BCN7712
CAS No.:19132-81-1
- Fmoc-Tyr(HPO3Bzl)-OH
Catalog No.:BCC3565
CAS No.:191348-16-0
- 3-Benzoylpropionic acid
Catalog No.:BCN1928
CAS No.:2051-95-8
- AGN 195183
Catalog No.:BCC5419
CAS No.:191469-29-1
- LY 379268
Catalog No.:BCC7368
CAS No.:191471-52-0
- Isoficusin A
Catalog No.:BCN6865
CAS No.:1914963-20-4
- 2-Hydroxyxanthone
Catalog No.:BCN7545
CAS No.:1915-98-6
- Epieriocalyxin A
Catalog No.:BCN1180
CAS No.:191545-24-1
Relaxant effect of YM976, a novel phosphodiesterase 4 inhibitor, on bovine tracheal smooth muscle.[Pubmed:12787831]
Eur J Pharmacol. 2003 May 30;470(1-2):57-64.
Effects of 4-(3-chlorophenyl)-1,7-diethylpyrido[2,3-d]pyrimidin-2(1H)-one (YM976), a novel and selective phosphodiesterase type 4 inhibitor, on tension and adenosine 3',5'-cyclic monophosphate (cAMP) content of bovine tracheal smooth muscle were compared with those of rolipram and theophylline. YM976, rolipram and theophylline relaxed the tracheal preparations contracted with histamine in a concentration-dependent manner. The relaxant effects of YM976 and rolipram were more potent than those of theophylline. These phosphodiesterase inhibitors-induced relaxations were dramatically diminished when tracheal smooth muscle was contracted with methacholine instead of histamine. Pretreatment of the tracheal preparations with YM976 (10 microM) or rolipram (10 microM), but not with theophylline (1 mM), shifted the concentration-response curves for contractile responses to histamine; however, the same procedure failed to affect concentration-response relationships for methacholine-induced contractions. At 1 and 10 microM, both YM976 and rolipram increased the tissues cAMP content. These results suggest that YM976 relaxes tracheal smooth muscle, probably through the cAMP-dependent mechanism.
Studies on mechanisms of low emetogenicity of YM976, a novel phosphodiesterase type 4 inhibitor.[Pubmed:11504812]
J Pharmacol Exp Ther. 2001 Sep;298(3):1142-9.
YM976 is a novel and selective inhibitor of phosphodiesterase type 4 (PDE4) with a different chemical structure from rolipram. Orally administered YM976 showed anti-inflammatory activity (ED(50) = 2.8 mg/kg) similar to rolipram (3.5 mg/kg). On the other hand, the emetogenicity of YM976, one of the main adverse effects of PDE4 inhibitors, was lower (maximal non-emetic dose = 10 mg/kg) than that of rolipram (1 mg/kg). The reasons for this low emetogenicity of YM976 remain unclear, and the present study endeavored to elucidate the mechanisms. Candidates for the possible mechanisms included 1) PDE4 subtype selectivity, 2) binding affinity for HAR-conformation, and 3) brain penetration. YM976 exhibited affinity for high affinity for rolipram-conformation (HAR-conformation) (IC(50) = 2.6 nM) identical to that of rolipram (1.2 nM), and failed to show significant selectivity for the individual PDE4 subtype. These results suggested that neither subtype selectivity nor the affinity for HAR-conformation may be related to the low emetogenicity of YM976. YM976 showed a minor effect on reserpine-induced hypothermia, in contrast to rolipram. To estimate brain penetration, we then measured cAMP contents in peripheral tissues (peritoneal macrophages) and in the brain. YM976 increased the cAMP content of peritoneal macrophages, but caused no significant increase in brain cAMP levels, while rolipram elevated the cAMP content of both tissues at the same dose. In conclusion, YM976 shows an apparent dissociation between its anti-inflammatory effects and emetogenicity, perhaps because of the poor brain penetration.
A novel phosphodiesterase type 4 inhibitor, YM976 (4-(3-chlorophenyl)-1,7-diethylpyrido[2,3-d]pyrimidin-2(1H)-one), with little emetogenic activity.[Pubmed:10991987]
J Pharmacol Exp Ther. 2000 Oct;295(1):255-60.
We synthesized a novel phosphodiesterase type 4 (PDE4) inhibitor, YM976, that is structurally different from the other PDE4 inhibitors like rolipram. In the present study, the pharmacological profile of YM976 was investigated. YM976 exhibited a strong and competitive inhibition against PDE4 purified from human peripheral leukocytes with an IC(50) of 2.2 nM. IC(50) values of rolipram and RP73401 were 820 and 0.43 nM, respectively. Test compounds had no effects on the other PDE isozymes, PDE1, -2, -3, and -5. YM976 potentiated prostaglandin E(2)-induced cAMP accumulation in a human mononuclear cell line, U937, and inhibited tumor necrosis factor-alpha production from human peripheral blood mononuclear cells stimulated by lipopolysaccharide. Anti-inflammatory activities of PDE4 inhibitors were compared in rat carrageenan-induced pleurisy models. YM976, rolipram, and RP73401 inhibited the cell infiltration into the pleural cavity with oral ED(30) values of 9.1, 10, and 7.4 mg/kg, respectively. YM976 produced no emesis up to 10 mg/kg, whereas rolipram and RP73401 induced emesis at oral doses of 3 mg/kg. To evidence the dissociation of anti-inflammatory activity from emesis, the anti-inflammatory effect of YM976 was examined in ferrets. YM976 dose dependently reduced carrageenan-induced leukocyte infiltration at the doses of 1, 3, and 10 mg/kg, p.o. On the other hand, rolipram failed to show obvious inhibition at doses that do not induce emesis. In conclusion, YM976 is a novel and orally active PDE4 inhibitor and possesses a good separation of emetogenicity from anti-inflammatory activity.


