AGN 195183RARα agonist CAS# 191469-29-1 |
- AM580
Catalog No.:BCC5373
CAS No.:102121-60-8
- AGN 194310
Catalog No.:BCC5416
CAS No.:229961-45-9
- Palovarotene
Catalog No.:BCC4185
CAS No.:410528-02-8
- AGN 205728
Catalog No.:BCC5418
CAS No.:859498-05-8
- AGN 196996
Catalog No.:BCC5417
CAS No.:958295-17-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 191469-29-1 | SDF | Download SDF |
| PubChem ID | 9977495 | Appearance | Powder |
| Formula | C22H23F2NO3 | M.Wt | 387.42 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
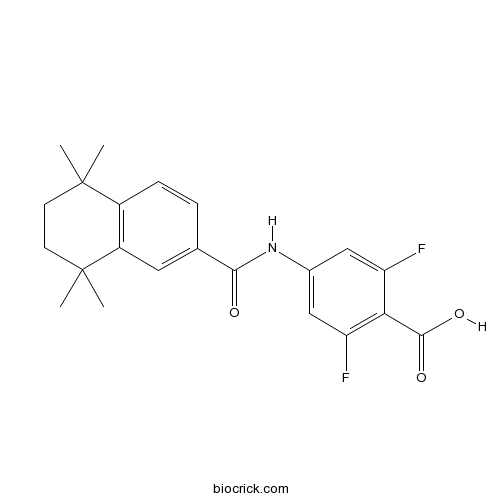
| Chemical Name | 2,6-difluoro-4-[(5,5,8,8-tetramethyl-6,7-dihydronaphthalene-2-carbonyl)amino]benzoic acid | ||
| SMILES | CC1(CCC(C2=C1C=CC(=C2)C(=O)NC3=CC(=C(C(=C3)F)C(=O)O)F)(C)C)C | ||
| Standard InChIKey | WTKJHVPWANEVRO-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C22H23F2NO3/c1-21(2)7-8-22(3,4)15-9-12(5-6-14(15)21)19(26)25-13-10-16(23)18(20(27)28)17(24)11-13/h5-6,9-11H,7-8H2,1-4H3,(H,25,26)(H,27,28) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

AGN 195183 Dilution Calculator

AGN 195183 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5812 mL | 12.9059 mL | 25.8118 mL | 51.6236 mL | 64.5295 mL |
| 5 mM | 0.5162 mL | 2.5812 mL | 5.1624 mL | 10.3247 mL | 12.9059 mL |
| 10 mM | 0.2581 mL | 1.2906 mL | 2.5812 mL | 5.1624 mL | 6.4529 mL |
| 50 mM | 0.0516 mL | 0.2581 mL | 0.5162 mL | 1.0325 mL | 1.2906 mL |
| 100 mM | 0.0258 mL | 0.1291 mL | 0.2581 mL | 0.5162 mL | 0.6453 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
AGN 195183 is a specific retinoic acid receptor alpha (RARα) agonist [1]. Its IC50 value hasn’t been determined.
Retinoic acid (RA) plays a key role in maintenance of certain tissues, normal development and growth. The action of RA is mediated in part by three nuclear receptors (RARa, -β, and -γ) [2].
Treatment with AGN 195183 at high doses raised serum triglyceride levels in both nonnephritic rats and nephritic rats, whereas low doses did not affect the serum triglyceride level [3].
In a rat model of mesangioproliferative glomerulonephritis, RA reduces glomerular injury [4]. In glomerulonephritis (Thy-GN), the expression of receptors of glomerular retinoid was differentially regulated. RARα was expressed at a markedly greater level in nephritic glomeruli than in nonnephritic controls. Treatment with AGN 195183 dose-dependently normalized the expression of glomerular RARα gene. The number of macrophages/monocytes per glomerular cross-section was greater in vehicle-treated nephritic rats than in controls. Treatment with AGN 195183 almost normalized this number of glomerular monocytes/macrophages. AGN 195183 at higher dose lowered glomerular ED-1+ cells more than the drug at lower doses. The expression of TGFβ1 was significantly greater in the glomeruli of nephritic rats than in controls. AGN 195183 significantly decreased TGFβ1 gene expression in nephritic glomeruli. AGN 195183 at the higher dose decreased glomerular TGFβ1 more pronounced than the drug at low dose [3].
References:
[1]. Zhou TB, Drummen GPC, Qin YH. The controversial role of retinoic Acid in fibrotic diseases: analysis of involved signaling pathways. International journal of molecular sciences, 2012, 14(1): 226-243.
[2]. Lufkin T, Lohnes D, Mark M, et al. High postnatal lethality and testis degeneration in retinoic acid receptor alpha mutant mice. Proceedings of the National Academy of Sciences, 1993, 90(15): 7225-7229.
[3]. Schaier M, Liebler S, Schade K, et al. Retinoic acid receptor α and retinoid X receptor specific agonists reduce renal injury in established chronic glomerulonephritis of the rat. Journal of molecular medicine, 2004, 82(2): 116-125.
[4]. Wagner J, Dechow C, Morath C, et al. Retinoic acid reduces glomerular injury in a rat model of glomerular damage. Journal of the American Society of Nephrology, 2000, 11(8): 1479-1487.
- 3-Benzoylpropionic acid
Catalog No.:BCN1928
CAS No.:2051-95-8
- Fmoc-Tyr(HPO3Bzl)-OH
Catalog No.:BCC3565
CAS No.:191348-16-0
- Ursolic aldehyde
Catalog No.:BCN7712
CAS No.:19132-81-1
- 6-Deoxy-3-O-methyl-beta-allopyranosyl(1-4)-beta-cymaronic acid delta-lactone
Catalog No.:BCN1514
CAS No.:19131-13-6
- 1-Deoxynojirimycin
Catalog No.:BCN1032
CAS No.:19130-96-2
- Boc-Asp(OtBu)-OH.DCHA
Catalog No.:BCC3369
CAS No.:1913-12-8
- C 75
Catalog No.:BCC2386
CAS No.:191282-48-1
- YM 976
Catalog No.:BCC7190
CAS No.:191219-80-4
- Pramipexole 2HCl Monohydrate
Catalog No.:BCC4466
CAS No.:191217-81-9
- Solasonine
Catalog No.:BCN2302
CAS No.:19121-58-5
- Atrazine
Catalog No.:BCC8838
CAS No.:1912-24-9
- Telithromycin
Catalog No.:BCC5273
CAS No.:191114-48-4
- LY 379268
Catalog No.:BCC7368
CAS No.:191471-52-0
- Isoficusin A
Catalog No.:BCN6865
CAS No.:1914963-20-4
- 2-Hydroxyxanthone
Catalog No.:BCN7545
CAS No.:1915-98-6
- Epieriocalyxin A
Catalog No.:BCN1180
CAS No.:191545-24-1
- 2-Deacetoxytaxinine B
Catalog No.:BCN1181
CAS No.:191547-12-3
- Trimethylgallic acid methyl ester
Catalog No.:BCN3369
CAS No.:1916-07-0
- SIB 1553A hydrochloride
Catalog No.:BCC6284
CAS No.:191611-89-9
- Pomalidomide (CC-4047)
Catalog No.:BCC2246
CAS No.:19171-19-8
- Tenacissoside G
Catalog No.:BCN4682
CAS No.:191729-43-8
- Tenacissoside I
Catalog No.:BCN4681
CAS No.:191729-44-9
- Tenacissoside H
Catalog No.:BCN2570
CAS No.:191729-45-0
- Lenalidomide (CC-5013)
Catalog No.:BCC2245
CAS No.:191732-72-6
Complexes of DNA bases and Watson-Crick base pairs interaction with neutral silver Agn (n = 8, 10, 12) clusters: a DFT and TDDFT study.[Pubmed:28325114]
J Biomol Struct Dyn. 2018 Mar;36(4):1050-1062.
We study the binding of the neutral Agn (n = 8, 10, 12) to the DNA base-adenine (A), guanine (G) and Watson-Crick -adenine-thymine, guanine-cytosine pairs. Geometries of complexes were optimized at the DFT level using the hybrid B3LYP functional. LANL2DZ effective core potential was used for silver and 6-31 + G(**) was used for all other atoms. NBO charges were analyzed using the Natural population analysis. The absorption properties of Agn-A,G/WC complexes were also studied using time-dependent density functional theory. The absorption spectra for these complexes show wavelength in the visible region. It was revealed that silver clusters interact more strongly with WC pairs than with isolated DNA complexes. Furthermore, it was found that the electronic charge transferred from silver to isolated DNA clusters are less than the electronic charge transferred from silver to the Agn-WC complexes. The vertical ionization potential, vertical electron affinity, hardness, and electrophilicity index of Agn-DNA/WC complexes have also been discussed.
Decursin in Angelica gigas Nakai (AGN) Enhances Doxorubicin Chemosensitivity in NCI/ADR-RES Ovarian Cancer Cells via Inhibition of P-glycoprotein Expression.[Pubmed:27605402]
Phytother Res. 2016 Dec;30(12):2020-2026.
Angelica gigas Nakai (AGN, Korean Dang-gui) is traditionally used for the treatment of various diseases including cancer. Here, we investigated multidrug-resistant phenotype-reversal activities of AGN and its compounds (decursin, ferulic acid, and nodakenin) in doxorubicin-resistant NCI/ADR-RES ovarian cancer cells. Our results showed that a combination of doxorubicin with either AGN or decursin inhibited a proliferation of NCI/ADR-RES cells. These combinations increased the number of cells at sub-G1 phase when cells were stained with Annexin V-fluorescein isothiocyanate. We also found that these combinations activated caspase-9, caspase-8, and caspase-3 and increased cleaved PARP level. Moreover, an inhibition of P-glycoprotein expression by either AGN or decursin resulted in a reduction of its activity in NCI/ADR-RES cells. Therefore, our data demonstrate that decursin in AGN inhibits doxorubicin-resistant ovarian cancer cell proliferation and induces apoptosis in the presence of doxorubicin via blocking P-glycoprotein expression. Therefore, AGN would be a potentially novel treatment option for multidrug-resistant tumors by sensitizing to anticancer agents. Copyright (c) 2016 John Wiley & Sons, Ltd.
Anti-cancer and other bioactivities of Korean Angelica gigas Nakai (AGN) and its major pyranocoumarin compounds.[Pubmed:22583405]
Anticancer Agents Med Chem. 2012 Dec;12(10):1239-54.
Korean Angelica gigas Nakai (AGN) is a major medicinal herb used in Asian countries such as Korea and China. Traditionally, its dried root has been used to treat anemia, pain, infection and articular rheumatism in Korea, most often through boiling in water to prepare the dosage forms. The pyranocoumarin compound decursin and its isomer decursinol angelate (DA) are the major chemical components in the alcoholic extracts of the root of AGN. The in vitro anti-tumor activities of decursin and/or DA against prostate cancer, lung cancer, breast cancer, colon cancer, bladder cancer, sarcoma, myeloma and leukemia have been increasingly reported in the past decade whereas the in vivo efficacy in mouse models was established only for a few organ sites. Preliminary pharmacokinetic studies by us and others in rodent models indicated that decursinol (DOH), which has much less in vitro direct anticancer activities by itself, is the major and rapid in vivo hydrolysis metabolite of both decursin and DA. Besides decursin, DA and DOH, other chemical components in AGN such as polysaccharides and polyacetylenes have been reported to exert anti-cancer and anti-inflammation activities as well. We systematically reviewed the published literature on the anti-cancer and other bio-activities effects of AGN extract and decursin, DA and DOH, as well as other chemicals identified from AGN. Although a number of areas are identified that merit further investigation, one critical need is first-in-human studies of the pharmacokinetics of decursin/DA to determine whether humans differ from rodents in absorption and metabolism of these compounds.
Usefulness of Chromogenic CromoCen(R) AGN agar medium for the identification of the genus Aeromonas: Assessment of faecal samples.[Pubmed:22561188]
J Microbiol Methods. 2012 Aug;90(2):100-4.
Selective screening media for the detection and identification of Aeromonas strains are needed to guide primary isolation procedures in the clinical laboratory. This study compared the selective CromoCen(R) AGN chromogenic agar medium for the detection and identification of Aeromonas strains that were isolated from various samples against the conventional selective agar media that are commonly used for the isolation of this organism in food, environmental and clinical samples. The Miles and Misra and ecometric methods were used to evaluate the microbiological performance of CromoCen(R) AGN chromogenic agar medium, which was shown to be satisfactory. A total of 14 reference Aeromonas strains, 44 wild strains and 106 clinical stool specimens were examined using both non-chromogenic selective agars that are commonly used for Aeromonas isolation and CromoCen(R) AGN agar. The latter exhibited 94.73% sensitivity and 100% specificity for the various samples. On CromoCen(R) AGN agar medium, Aeromonas formed colonies with light green, greenish and salmon pigments with or without a surrounding wide transparent zone (halo) of 2-3mm in diameter around the entire border. This medium is recommended for the isolation and potential identification of the Aeromonas genus.


