Asarum heterotropoides
Asarum heterotropoides
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Asarum heterotropoides
- Cat.No. Product Name CAS Number COA
-
BCN9084
(-)-Sesamin13079-95-3
Instructions

-
BCN2290
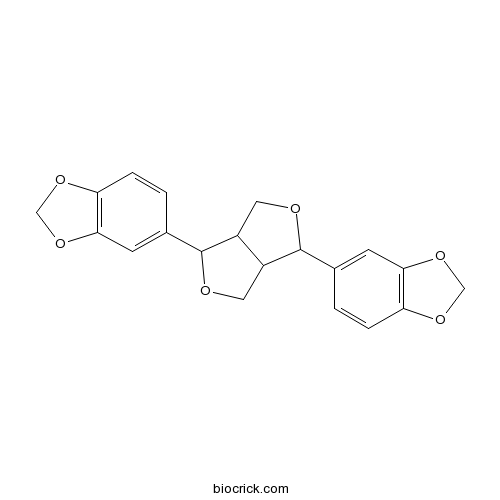
(-)-Asarinin133-04-0
Instructions
-
BCN2902
Aristolochic acid D17413-38-6
Instructions

-
BCN6455
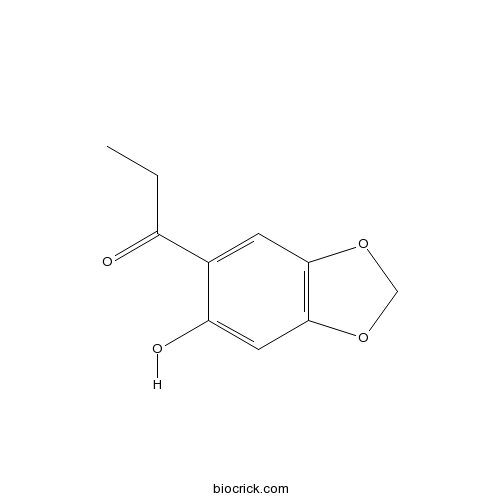
Kakuol18607-90-4
Instructions
-
BCN2730
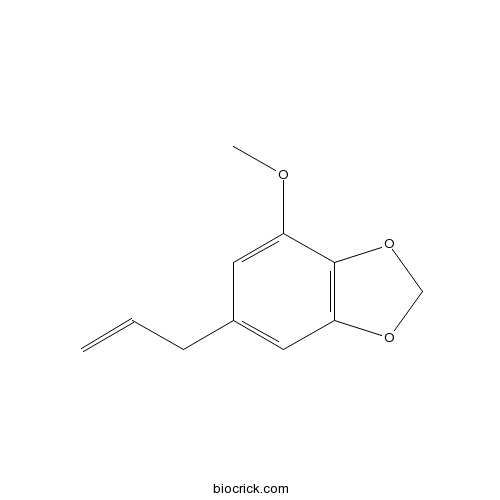
Myristicin607-91-0
Instructions
-
BCN8243
Methyl Kakuol70342-29-9
Instructions

-
BCC8275
(1R)-(+)-Alpha-Pinene7785-70-8
Instructions

-
BCN4074
Methyleugenol93-15-2
Instructions

Composition of Asarum heterotropoides var. mandshuricum radix oil from different extraction methods and activities against human body odor-producing bacteria.[Pubmed: 28911620]
None
Phenanthrene derivatives from roots and rhizomes of Asarum heterotropoides var. mandshuricum.[Pubmed: 28126415]
None
Chemical Constituents from the Roots and Rhizomes of Asarum heterotropoides var. mandshuricum and the In Vitro Anti-Inflammatory Activity.[Pubmed: 28098805]
None
Acaricidal activity of Asarum heterotropoides root-derived compounds and hydrodistillate constitutes toward Dermanyssus gallinae (Mesostigmata: Dermanyssidae).[Pubmed: 26708137]
The acaricidal activity of Asarum heterotropoides root-derived principles, methyleugenol, safrole, 3-carene, α-asarone, pentadecane and A. heterotropoides root steam distillate constituents was tested against poultry red mites Dermanyssus gallinae (De Geer). All active principles were identified by spectroscopic analysis. Results were compared with those of two conventional acaricides, benzyl benzoate and N,N-diethyl-3-methylbenzamide (DEET). Methyleugenol (24 h LC50 = 0.57 µg/cm(2)) and safrole (24 h LC50 = 8.54 µg/cm(2)) were the most toxic compounds toward D. gallinae, followed by 3,4,5-trimethoxytoluene, 3,5-dimethoxytoluene, estragole, α-terpineol, verbenone, eucarvone, linalool, and terpinen-4-ol (LC50 = 15.65-27.88 µg/cm(2)). Methyleugenol was 16.7× and 11.0× more toxic than benzyl benzoate (LC50 = 9.52 μg/cm(2)) and DEET (LC50 = 6.28 μg/cm(2)), respectively; safrole was 1.1× and 0.73× more toxic. Asarum heterotropoides root-derived materials, particularly methyleugenol and safrole, merit further study as potential acaricides. Global efforts to reduce the level of highly toxic synthetic acaricides in indoor environments justify further studies on A. heterotropoides root extract and steam distillate preparations containing the active constituents described as potential contact-action fumigants for the control of mites.
[Qualitative and quantitative analysis of dodecatetraenamides A, B in Asari Radix et Rhizoma].[Pubmed: 26137692]
To develop an analytic method for qualitative and quantitative analysis of dodecatetraenamides A, B in 42 samples of two official species of Asari Radix et Rhizoma( ARR) (37 samples of Asarum heterotropoides var. mandshuricum with different collection time and 5 samples of Asarum sieboldiivar. seoulense). The HPLC-IT-TOF-MS/MS methods for the qualitative and UPLC-PDA methods for the quantitative analysis were established. Dodecatetraenamides A, B were identified by comparing the retention time, UV absorption spectrum and quasi-molecular ion peak [ M + H]+ with the reference compound using HPLC-IT-TOF-MS/MS. The content of dodecatetraenamides A and B in ARR were determined by UPLC-PDA. The separation was successfully carried out on a ACQUITY UPLC BEH C18 (2.1 mm x 100 mm, 1.7 µm) column eluted with mobile phases of water (A) and acetonitrile (B) in gradient program (0-3 min, 35% B; 3-5 min, 35%-36% B; 5-6 min, 36%-43% B; 6 min-11 min 43% B; 11-12 min, 43%-100% B). The column temperature was 45 °C, and the detection wavelength was set at 254 nm. The flow rate was 0.6 mL · min(-1). On one level mass spectrometry scanning, the results showed that the quasi-molecular ion [M + H] + of both dodecatetraenamides A and B were m/z 248.20. The quantitative method with UPLC-PDA has made the baseline separation of the constituents, which were reported as mixtures in the most literatures. The average recovery of dodecatetraenamides A and B were 97.90% and 99.86%, the relative standard deviation were 0.4% and 1.1%, respectively. The contents of dodecatetraenamides A, B in all ARR samples was in the range of 0.11-3.89 and 0.24-6.65 mg · g(-1). Their contents reduced with the extension of storage time. Compared with the samples of 2013, the average content of the two constituents in the samples collected in year 2002-2003 reduced 34% and 36%, respectively (P < 0.05). Compared the A. sieboldii var. seoulense and A. heterotropoides var. mandshuricum with the same collective time and production area, the average contents of the two constituents in latter were up to (1.59 ± 0.75) mg · g(-1) and (2.90 ± 1.17) mg · g(-1), respectively, significantly higher than that in A. sieboldii var. seoulense (dodecatetraenamide A were (0.78 ± 0.52) mg · g(-1), dodecatetraenamide B were (1.69 ± 0.83) mg · g(-1)) (P < 0.05). The content of the dodecatetraenamide A in overground part was in the range of 0.11-0.33 mg · g(-1), dodecatetraenamide B was 0. 24-0.60 mg · g(-1), which were much lower than that of the underground part of ARR (dodecatetraenamide A was in the range of 0.73-3.89 mg · g(-1), dodecatetraenamide B was 2.11-6.24 mg · g(-1)). The method was certified to be simple, accurate and reliable and could be used for qualitative and quantitative analysis of dodecatetraenamide A and B in different species of ARR, also can be used for the comprehensive quality control of traditional Chinese medicine, Asari Radix et Rhizoma.
Effect of the fragrance inhalation of essential oil from Asarum heterotropoides on depression-like behaviors in mice.[Pubmed: 25881143]
Psychological stressors may cause affective disorders, such as depression and anxiety, by altering expressions of corticotropin releasing factor (CRF), serotonin (5-HT), and tyrosine hydroxylase (TH) in the brain. This study investigated the effects of essential oil from Asarum heterotropoides (EOAH) on depression-like behaviors and brain expressions of CRF, 5-HT, and TH in mice challenged with stress.
Simultaneous determination of twenty-two components in Asari Radix et Rhizoma by ultra performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry.[Pubmed: 25513865]
Asari Radix et Rhizoma is a herbal medicine for the treatment of common cold, rhinitis, etc. An ultra performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry method has been established for the rapid analysis of 22 components in 27 samples from the raw materials of Asari Radix et Rhizoma and an adulterant. A total of 5 lignans, 5 essential oils, 3 aristolochic acids, 4 alkamides, and 5 flavanoids were identified by co-chromatography of samples extracts and comparison of the retention time, UV spectra, characteristic molecular ions, and fragment ions with those of authentic standards, or tentatively identified by MS/MS determination along with MassFragment software. Moreover, the method was validated for the simultaneous quantification and semi-quantification of 20 components. The samples from Asarum heterotropoides var. mandshuricum differed in the quantity of 2-methoxyl-4,5-methylenedioxypropiophenone and kakuol from those of Asarum sieboldii var. seoulense, and the chemical difference was supported by principal component analysis and orthogonal partial least squared discriminant analysis based on dataset obtained from UHPLC-QTOF/MS. In comparison with the samples from the two medicinal Asarum species mentioned above, those from A. himalaicum differed in the quality and quantity of major compounds and contained higher amounts of aristolochic acid I.
[Composition analysis of volatile oil of Asarum heterotropoides var. mandshuricum extracted by three different methods].[Pubmed: 24620691]
To study the differences of the constituents of the volatile oil from Asarum heterotropoides var. mandshuricum with three different extracted methods.


