MyristicinCAS# 607-91-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 607-91-0 | SDF | Download SDF |
| PubChem ID | 4276 | Appearance | Light yellow liquid |
| Formula | C11H12O3 | M.Wt | 192.21 |
| Type of Compound | Phenylpropanoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in acetone, ethanol and methanol; insoluble in water | ||
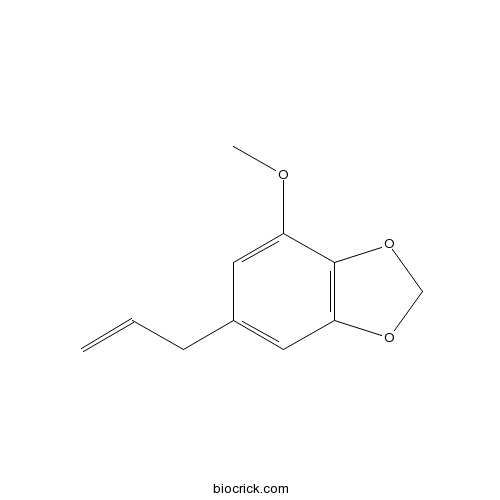
| Chemical Name | 4-methoxy-6-prop-2-enyl-1,3-benzodioxole | ||
| SMILES | COC1=CC(=CC2=C1OCO2)CC=C | ||
| Standard InChIKey | BNWJOHGLIBDBOB-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C11H12O3/c1-3-4-8-5-9(12-2)11-10(6-8)13-7-14-11/h3,5-6H,1,4,7H2,2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Myristicin has anti-cholinergic, antibacterial, and hepatoprotective effects, it also has anti-inflammatory properties related with its inhibition of NO, cytokines,chemokines, and growth factors in dsRNA-stimulated macrophages via the calcium pathway. Myristicin may antagonize the anxiolytic effects of midazolam, increase anxiety, and affect motor movements. Myristicin can induce apoptosis as characterised by alterations in the mitochondrial membrane potential, cytochrome c release, caspase-3 activation, PARP-cleavage and DNA fragmentation. |
| Targets | P450 (e.g. CYP17) | Bcl-2/Bax | Caspase | PARP | NO | IL Receptor | GABA Receptor | Antifection |
| In vitro | Anti-inflammatory effect of myristicin on RAW 264.7 macrophages stimulated with polyinosinic-polycytidylic acid.[Pubmed: 21991618]Molecules. 2011;16(8):7132-42.Myristicin (1-allyl-5-methoxy-3,4-methylenedioxybenzene) is an active aromatic compound found in nutmeg (the seed of Myristica fragrans), carrot, basil,cinnamon, and parsley. Myristicin has been known to have anti-cholinergic, antibacterial,and hepatoprotective effects, however, the effects of Myristicin on virus-stimulated macrophages are not fully reported. |
| In vivo | Evaluation of the anxiolytic properties of myristicin, a component of nutmeg, in the male Sprague-Dawley rat.[Pubmed: 21560973]AANA J. 2011 Apr;79(2):109-14.The purpose of this study was to investigate the anxiolytic effects of Myristicin, a major compound found in nutmeg, and its potential interaction with the gamma-aminobutyric acid (GABA(A)) receptor in male Sprague-Dawley rats.
|
| Kinase Assay | Myristicin from nutmeg induces apoptosis via the mitochondrial pathway and down regulates genes of the DNA damage response pathways in human leukaemia K562 cells.[Pubmed: 24792648]Chem Biol Interact. 2014 Jul 25;218:1-9.Myristicin, an allylbenzene, is a major active component of various spices, such as nutmeg and cinnamon, plants from the Umbelliferae family or in some essential oils, such as oils of clove or marjoram. Human exposure to Myristicin is low but widespread due to consumption of these spices and essential oils, added to food (e.g. cola drinks) or in traditional medicine. Occasionally high dose exposure occurs, leading to various clinical symptoms, however the molecular mechanisms underlying them are unknown.
Our previous studies revealed that Myristicin is not genotoxic and yet presented apoptotic activity.
|

Myristicin Dilution Calculator

Myristicin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.2026 mL | 26.0132 mL | 52.0264 mL | 104.0529 mL | 130.0661 mL |
| 5 mM | 1.0405 mL | 5.2026 mL | 10.4053 mL | 20.8106 mL | 26.0132 mL |
| 10 mM | 0.5203 mL | 2.6013 mL | 5.2026 mL | 10.4053 mL | 13.0066 mL |
| 50 mM | 0.1041 mL | 0.5203 mL | 1.0405 mL | 2.0811 mL | 2.6013 mL |
| 100 mM | 0.052 mL | 0.2601 mL | 0.5203 mL | 1.0405 mL | 1.3007 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Sesamin
Catalog No.:BCN4123
CAS No.:607-80-7
- FC 131
Catalog No.:BCC7917
CAS No.:606968-52-9
- 3-Hydroxy-1,5-diphenyl-1-pentanone
Catalog No.:BCN3536
CAS No.:60669-64-9
- Isopicropodophyllone
Catalog No.:BCN8316
CAS No.:60660-50-6
- HOSu
Catalog No.:BCC2845
CAS No.:6066-82-6
- 3-n-Butylphthalide
Catalog No.:BCN2381
CAS No.:6066-49-5
- Dihydromollugin
Catalog No.:BCN8247
CAS No.:60657-93-4
- Danaidone
Catalog No.:BCN1966
CAS No.:6064-85-3
- Bifonazole
Catalog No.:BCC4766
CAS No.:60628-96-8
- MEK162 (ARRY-162, ARRY-438162)
Catalog No.:BCC1148
CAS No.:606143-89-9
- AZD6244 (Selumetinib)
Catalog No.:BCC3624
CAS No.:606143-52-6
- Momor-cerebroside I
Catalog No.:BCN4120
CAS No.:606125-07-9
- Physoperuvine
Catalog No.:BCN1402
CAS No.:60723-27-5
- SB 772077B dihydrochloride
Catalog No.:BCC6116
CAS No.:607373-46-6
- AZ 10606120 dihydrochloride
Catalog No.:BCC6005
CAS No.:607378-18-7
- Canthin-6-one N-oxide
Catalog No.:BCN2992
CAS No.:60755-87-5
- 2-Hydroxy-1,8-cineole
Catalog No.:BCN4121
CAS No.:60761-00-4
- ent-Kauran-17,19-dioic acid
Catalog No.:BCN4122
CAS No.:60761-79-7
- Meloside A
Catalog No.:BCN2278
CAS No.:60767-80-8
- Ethyl 4-methoxysalicylate
Catalog No.:BCN3499
CAS No.:35031-00-6
- A 77-01
Catalog No.:BCC1318
CAS No.:607737-87-1
- SB742457
Catalog No.:BCC5058
CAS No.:607742-69-8
- Quercetin 3-O-beta-D-glucose-7-O-beta-D-gentiobioside
Catalog No.:BCN7821
CAS No.:60778-02-1
- Berbamine hydrochloride
Catalog No.:BCN2400
CAS No.:6078-17-7
Evaluation of the anxiolytic properties of myristicin, a component of nutmeg, in the male Sprague-Dawley rat.[Pubmed:21560973]
AANA J. 2011 Apr;79(2):109-14.
The purpose of this study was to investigate the anxiolytic effects of Myristicin, a major compound found in nutmeg, and its potential interaction with the gamma-aminobutyric acid (GABA(A)) receptor in male Sprague-Dawley rats. Nutmeg has traditionally been used as a spice in food preparation and as an herbal remedy in the treatment of many medical conditions, including anxiety. Fifty-five rats were divided equally into 5 groups: control (vehicle); Myristicin; midazolam (positive control); flumazenil and Myristicin; and midazolam and Myristicin. The behavioral component of anxiety was examined by using the elevated plus-maze (open-arm and closed-arm times) along with analysis of gross and fine motor movements. Data analysis was performed using a 2-tailed multivariate analysis of variance (MANOVA) and least significant difference post-hoc test. Our data suggest that Myristicin does not decrease anxiety by modulation of the GABA(A) receptor but may promote anxiogenesis. When Myristicin was combined with midazolam, an antagonist-like effect similar to the flumazenil and Myristicin combination was exhibited by a decrease in anxiolysis compared with the midazolam-only group. Myristicin may antagonize the anxiolytic effects of midazolam, increase anxiety, and affect motor movements.
Anti-inflammatory effect of myristicin on RAW 264.7 macrophages stimulated with polyinosinic-polycytidylic acid.[Pubmed:21991618]
Molecules. 2011;16(8):7132-42.
Myristicin (1-allyl-5-methoxy-3,4-methylenedioxybenzene) is an active aromatic compound found in nutmeg (the seed of Myristica fragrans), carrot, basil,cinnamon, and parsley. Myristicin has been known to have anti-cholinergic, antibacterial,and hepatoprotective effects, however, the effects of Myristicin on virus-stimulated macrophages are not fully reported. In this study, the anti-inflammatory effect of Myristicin on double-stranded RNA (dsRNA)-stimulated macrophages was examined. Myristicin did not reduce the cell viability of RAW 264.7 mouse macrophages at concentrations of up to 50 muM. Myristicin significantly inhibited the production of calcium, nitric oxide (NO),interleukin (IL)-6, IL-10, interferon inducible protein-10, monocyte chemotactic protein(MCP)-1, MCP-3, granulocyte-macrophage colony-stimulating factor, macrophage inflammatory protein (MIP)-1alpha, MIP-1beta, and leukemia inhibitory factor in dsRNA[polyinosinic-polycytidylic acid]-induced RAW 264.7 cells (P < 0.05). In conclusion,Myristicin has anti-inflammatory properties related with its inhibition of NO, cytokines,chemokines, and growth factors in dsRNA-stimulated macrophages via the calcium pathway.
Myristicin from nutmeg induces apoptosis via the mitochondrial pathway and down regulates genes of the DNA damage response pathways in human leukaemia K562 cells.[Pubmed:24792648]
Chem Biol Interact. 2014 Jul 25;218:1-9.
Myristicin, an allylbenzene, is a major active component of various spices, such as nutmeg and cinnamon, plants from the Umbelliferae family or in some essential oils, such as oils of clove or marjoram. Human exposure to Myristicin is low but widespread due to consumption of these spices and essential oils, added to food (e.g. cola drinks) or in traditional medicine. Occasionally high dose exposure occurs, leading to various clinical symptoms, however the molecular mechanisms underlying them are unknown. Our previous studies revealed that Myristicin is not genotoxic and yet presented apoptotic activity. Therefore, in this work we assessed the apoptotic mechanisms induced by Myristicin in human leukaemia cells. In order to gain further insight on the potential of Myristicin to modulate gene expression we also analysed alterations in expression of 84 genes associated with the DNA damage response pathway. The results obtained show that Myristicin can induce apoptosis as characterised by alterations in the mitochondrial membrane potential, cytochrome c release, caspase-3 activation, PARP-cleavage and DNA fragmentation. The gene expression profile revealed an overall down regulation of DNA damage response genes after exposure to Myristicin, with significant under-expression of genes associated with nucleotide excision repair (ERCC1), double strand break repair (RAD50, RAD51) and DNA damage signalling (ATM) and stress response (GADD45A, GADD45G). On the whole, we demonstrate that Myristicin can alter mitochondrial membrane function, induce apoptosis and modulate gene expression in human leukaemia K562 cells. This study provides further detail on the molecular mechanisms underlying the biological activity of Myristicin.


