FC 131CXCR4 antagonist CAS# 606968-52-9 |
- chroman 1
Catalog No.:BCC1480
CAS No.:1273579-40-0
- RKI-1447
Catalog No.:BCC1903
CAS No.:1342278-01-6
- Y-27632
Catalog No.:BCC4301
CAS No.:146986-50-7
- Y-27632 dihydrochloride
Catalog No.:BCC1273
CAS No.:129830-38-2
- GSK429286A
Catalog No.:BCC2532
CAS No.:864082-47-3
- SLx-2119
Catalog No.:BCC1954
CAS No.:911417-87-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 606968-52-9 | SDF | Download SDF |
| PubChem ID | 5275843 | Appearance | Powder |
| Formula | C36H47N11O6 | M.Wt | 729.84 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 2 mg/ml in water | ||
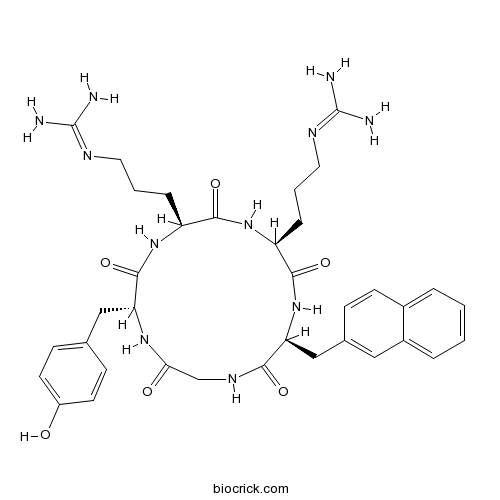
| Sequence | XGYRR (Modifications: Cyclic peptide, X = 2-Nal, Tyr-3 = D-Tyr] | ||
| Chemical Name | 2-[3-[(2S,5S,8S,14R)-5-[3-(diaminomethylideneamino)propyl]-14-[(4-hydroxyphenyl)methyl]-8-(naphthalen-2-ylmethyl)-3,6,9,12,15-pentaoxo-1,4,7,10,13-pentazacyclopentadec-2-yl]propyl]guanidine | ||
| SMILES | C1C(=O)NC(C(=O)NC(C(=O)NC(C(=O)NC(C(=O)N1)CC2=CC3=CC=CC=C3C=C2)CCCN=C(N)N)CCCN=C(N)N)CC4=CC=C(C=C4)O | ||
| Standard InChIKey | MBXBICVKLVYNKD-XFTNXAEASA-N | ||
| Standard InChI | InChI=1S/C36H47N11O6/c37-35(38)41-15-3-7-26-32(51)45-27(8-4-16-42-36(39)40)33(52)47-28(19-22-9-12-23-5-1-2-6-24(23)17-22)31(50)43-20-30(49)44-29(34(53)46-26)18-21-10-13-25(48)14-11-21/h1-2,5-6,9-14,17,26-29,48H,3-4,7-8,15-16,18-20H2,(H,43,50)(H,44,49)(H,45,51)(H,46,53)(H,47,52)(H4,37,38,41)(H4,39,40,42)/t26-,27-,28-,29+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | CXCR4 antagonist (IC50 = 126 nM). Displays anti-HIV activity in assays using NL4-3 and IIIB strains (EC50 = 21 nM for both strains). |

FC 131 Dilution Calculator

FC 131 Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 3-Hydroxy-1,5-diphenyl-1-pentanone
Catalog No.:BCN3536
CAS No.:60669-64-9
- Isopicropodophyllone
Catalog No.:BCN8316
CAS No.:60660-50-6
- HOSu
Catalog No.:BCC2845
CAS No.:6066-82-6
- 3-n-Butylphthalide
Catalog No.:BCN2381
CAS No.:6066-49-5
- Dihydromollugin
Catalog No.:BCN8247
CAS No.:60657-93-4
- Danaidone
Catalog No.:BCN1966
CAS No.:6064-85-3
- Bifonazole
Catalog No.:BCC4766
CAS No.:60628-96-8
- MEK162 (ARRY-162, ARRY-438162)
Catalog No.:BCC1148
CAS No.:606143-89-9
- AZD6244 (Selumetinib)
Catalog No.:BCC3624
CAS No.:606143-52-6
- Momor-cerebroside I
Catalog No.:BCN4120
CAS No.:606125-07-9
- MK-0773
Catalog No.:BCC1754
CAS No.:606101-58-0
- Homopterocarpin
Catalog No.:BCN4615
CAS No.:606-91-7
- Sesamin
Catalog No.:BCN4123
CAS No.:607-80-7
- Myristicin
Catalog No.:BCN2730
CAS No.:607-91-0
- Physoperuvine
Catalog No.:BCN1402
CAS No.:60723-27-5
- SB 772077B dihydrochloride
Catalog No.:BCC6116
CAS No.:607373-46-6
- AZ 10606120 dihydrochloride
Catalog No.:BCC6005
CAS No.:607378-18-7
- Canthin-6-one N-oxide
Catalog No.:BCN2992
CAS No.:60755-87-5
- 2-Hydroxy-1,8-cineole
Catalog No.:BCN4121
CAS No.:60761-00-4
- ent-Kauran-17,19-dioic acid
Catalog No.:BCN4122
CAS No.:60761-79-7
- Meloside A
Catalog No.:BCN2278
CAS No.:60767-80-8
- Ethyl 4-methoxysalicylate
Catalog No.:BCN3499
CAS No.:35031-00-6
- A 77-01
Catalog No.:BCC1318
CAS No.:607737-87-1
- SB742457
Catalog No.:BCC5058
CAS No.:607742-69-8
Rapid detection of the Fc gamma RIIA-H/R 131 ligand-binding polymorphism using an allele-specific restriction enzyme digestion (ASRED).[Pubmed:8960098]
J Immunol Methods. 1996 Nov 29;199(1):55-9.
A polymorphism of the gene for Fc gamma RIIA, arginine (R) or histidine (H) at position 131, alters the ability of the receptor to bind certain IgG subclasses. Identification of the Fc gamma RIIA-H/R 131 genotype has assumed increasing importance in disorders of host defense, immunohematologic diseases and systemic autoimmune disorders. We report a new method for determination of this genotype in which an allele-specific restriction enzyme site is introduced into an Fc gamma RIIA PCR product from genomic DNA, and polymorphism assignment is determined by restriction enzyme digestion followed by agarose gel electrophoresis. This method is more rapid, more reliable and less expensive than currently available methods.
Fc gamma RIIA H/R 131 polymorphism, subclass-specific IgG anti-heparin/platelet factor 4 antibodies and clinical course in patients with heparin-induced thrombocytopenia and thrombosis.[Pubmed:9002937]
Blood. 1997 Jan 15;89(2):370-5.
The explanation why only a subset of patients with heparin-induced thrombocytopenia (HIT) develop clinically apparent thromboses (HITT) remains uncertain. It has been proposed that platelet activation induced by cross-linking of Fc gamma RIIA by anti-heparin/platelet factor 4 (PF4) antibodies is central to the pathogenesis of thrombosis. The observation that a common functional polymorphism of Fc gamma RIIA, involving either an arginine (R) or histidine (H) at amino acid 131, may underlie disease susceptibility prompted us to investigate the prevalence of receptor isoforms in patients with HIT and HITT. Furthermore, because these isoforms reportedly differ in their avidity for immune complexes containing human IgG2, we also analyzed sera from patients with HIT and HITT for the prevalence of various subclass-specific IgG anti-heparin/PF4 antibodies. No difference in the allele frequency of Fc gamma RIIA-H131 or R131 was identified among 13 patients with HIT or 23 with HITT compared with 102 controls (chi 2 = 1.21, P = .8). Furthermore, although most patients had IgG2 antibodies (62%), IgG, was the predominant subclass in 30 of the 34 patients with IgG anti-heparin/PF4 antibodies and in 12 was the exclusive subclass found. Also, there was no association between the concordance of IgG2 anti-heparin/ PF4 antibodies and the expression of Fc gamma RIIA-H131 in patients with HITT compared with patients with thrombocytopenia alone. These results make it unlikely that the Fc gamma RIIA-H131 isoform or IgG2 anti-heparin/PF4 antibodies are required to develop HITT, suggesting that factors in addition to cross-linking of Fc gamma RIIA receptors contribute to the pathogenesis of thrombosis in patients with heparin-dependent antiplatelet: antibodies.
Stereoselective synthesis of [L-Arg-L/D-3-(2-naphthyl)alanine]-type (E)-alkene dipeptide isosteres and its application to the synthesis and biological evaluation of pseudopeptide analogues of the CXCR4 antagonist FC131.[Pubmed:15658852]
J Med Chem. 2005 Jan 27;48(2):380-91.
L,L-Type and L,D-type (E)-alkene dipeptide isosteres (EADIs) that have unnatural side chains at the alpha-position were synthesized by the combination of stereoselective aziridinyl ring-opening reactions and organozinc-copper-mediated anti-S(N)2' reactions toward a single substrate of gamma,delta-cis-gamma,delta-epimino (E)-alpha,beta-enoate. The utility of this methodology was demonstrated by the stereoselective synthesis of a set of diastereomeric EADIs of L-Arg-L/D-3-(2-naphthyl)alanine (Nal) that is contained in a small CXCR4 antagonist FC131 [cyclo(-D-Tyr-Arg-Arg-Nal-Gly-)]. Furthermore, a (Nal-Gly)-type EADI was synthesized by samarium diiodide (SmI(2))-induced reduction of a gamma-acetoxy-alpha,beta-enoate. Several FC131 analogues, in which these EADIs were inserted for reduction of their peptide character, were synthesized with analogues containing reduced amide-type dipeptide isosteres to investigate the importance of these amide bonds for anti-HIV and CXCR4-antagonistic activity.


