BifonazoleCAS# 60628-96-8 |
- RU 58841
Catalog No.:BCC1911
CAS No.:154992-24-2
- 17 alpha-propionate
Catalog No.:BCC1296
CAS No.:19608-29-8
- Spironolactone
Catalog No.:BCC4366
CAS No.:52-01-7
- MDV3100 (Enzalutamide)
Catalog No.:BCC1268
CAS No.:915087-33-1
- ARN-509
Catalog No.:BCC3724
CAS No.:956104-40-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 60628-96-8 | SDF | Download SDF |
| PubChem ID | 2378 | Appearance | Powder |
| Formula | C22H18N2 | M.Wt | 310.39 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 33.33 mg/mL (107.38 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
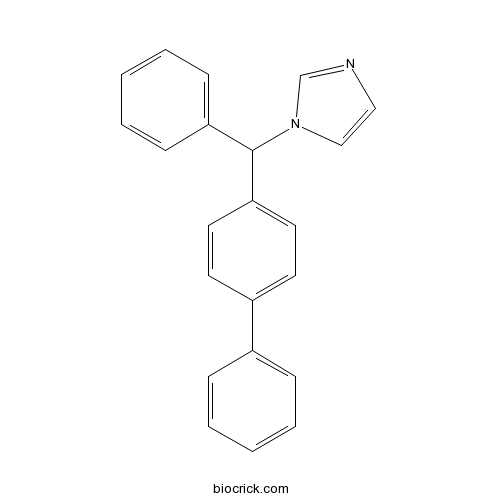
| Chemical Name | 1-[phenyl-(4-phenylphenyl)methyl]imidazole | ||
| SMILES | C1=CC=C(C=C1)C2=CC=C(C=C2)C(C3=CC=CC=C3)N4C=CN=C4 | ||
| Standard InChIKey | OCAPBUJLXMYKEJ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C22H18N2/c1-3-7-18(8-4-1)19-11-13-21(14-12-19)22(24-16-15-23-17-24)20-9-5-2-6-10-20/h1-17,22H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Bifonazole Dilution Calculator

Bifonazole Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.2218 mL | 16.1088 mL | 32.2175 mL | 64.4351 mL | 80.5438 mL |
| 5 mM | 0.6444 mL | 3.2218 mL | 6.4435 mL | 12.887 mL | 16.1088 mL |
| 10 mM | 0.3222 mL | 1.6109 mL | 3.2218 mL | 6.4435 mL | 8.0544 mL |
| 50 mM | 0.0644 mL | 0.3222 mL | 0.6444 mL | 1.2887 mL | 1.6109 mL |
| 100 mM | 0.0322 mL | 0.1611 mL | 0.3222 mL | 0.6444 mL | 0.8054 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Bifonazole
- MEK162 (ARRY-162, ARRY-438162)
Catalog No.:BCC1148
CAS No.:606143-89-9
- AZD6244 (Selumetinib)
Catalog No.:BCC3624
CAS No.:606143-52-6
- Momor-cerebroside I
Catalog No.:BCN4120
CAS No.:606125-07-9
- MK-0773
Catalog No.:BCC1754
CAS No.:606101-58-0
- Homopterocarpin
Catalog No.:BCN4615
CAS No.:606-91-7
- Cinnabarinic acid
Catalog No.:BCC7865
CAS No.:606-59-7
- Toyocamycin
Catalog No.:BCC8047
CAS No.:606-58-6
- 2,4'-Dihydroxybenzophenone
Catalog No.:BCN3358
CAS No.:606-12-2
- Pamabrom
Catalog No.:BCC1835
CAS No.:606-04-2
- Tirandamycin B
Catalog No.:BCN1862
CAS No.:60587-14-6
- DCEBIO
Catalog No.:BCC7060
CAS No.:60563-36-2
- P1075
Catalog No.:BCC7027
CAS No.:60559-98-0
- Danaidone
Catalog No.:BCN1966
CAS No.:6064-85-3
- Dihydromollugin
Catalog No.:BCN8247
CAS No.:60657-93-4
- 3-n-Butylphthalide
Catalog No.:BCN2381
CAS No.:6066-49-5
- HOSu
Catalog No.:BCC2845
CAS No.:6066-82-6
- Isopicropodophyllone
Catalog No.:BCN8316
CAS No.:60660-50-6
- 3-Hydroxy-1,5-diphenyl-1-pentanone
Catalog No.:BCN3536
CAS No.:60669-64-9
- FC 131
Catalog No.:BCC7917
CAS No.:606968-52-9
- Sesamin
Catalog No.:BCN4123
CAS No.:607-80-7
- Myristicin
Catalog No.:BCN2730
CAS No.:607-91-0
- Physoperuvine
Catalog No.:BCN1402
CAS No.:60723-27-5
- SB 772077B dihydrochloride
Catalog No.:BCC6116
CAS No.:607373-46-6
- AZ 10606120 dihydrochloride
Catalog No.:BCC6005
CAS No.:607378-18-7
Evaluation of efficacy and tolerability of four weeks bifonazole treatment after nail ablation with 40% urea in mild to moderate distal subungual onychomycosis.[Pubmed:26472342]
G Ital Dermatol Venereol. 2016 Feb;151(1):32-6. Epub 2015 Oct 16.
BACKGROUND: The aim of this study was to verify efficacy and tolerability of sequential therapy with 40% urea paste followed by 1% Bifonazole urea in mild to moderate distal subungual onychomycosis of the toenails. METHODS: It was an seven weeks open study. Sequential patients affected by mild to moderate distal subungual onychomycosis of the toenails agreed to apply on the affected nail 40% urea paste in occlusion overnight for the first three weeks, with gentle scraping with a spatula the following day, followed by 1% Bifonazole cream once a day for 4 weeks. Efficacy evaluation was based on mycology, clinical photography and investigator and patient assessment. Tolerability assessment included subjective and objective evaluations. RESULTS: The ten patients enrolled (mean age 57.5 years) completed the study. Onychomycosis was caused in nine cases by dermatophytes and by Scopulariopsis brevicaulis in one patient. At the end of the study, mycological examination was negative in all 10 patients. Clinical photographs showed a reduction of the percentage of the nail affected by onychomycosis in 8 cases, cure in 2 and considerable reduction of the nail thickness, already evident after 7 days. All patients reported to be satisfied by the treatment, which was judged easy to perform and well tolerated. CONCLUSIONS: Treatment with urea and Bifonazole is effective and well tolerated, and easy to do also by elderly patients.
Determination of bifonazole and identification of its photocatalytic degradation products using UPLC-MS/MS.[Pubmed:28186351]
Biomed Chromatogr. 2017 Sep;31(9).
The main goal of the presented work was to investigate the effect of ZnO or/and TiO2 on the stability of Bifonazole in solutions under UVA irradiation. To this end, a simple and reproducible UPLC method for the determination of Bifonazole in the presence of its photocatalytic degradation products was developed. Linearity was studied in the range of 0.0046-0.15 mg mL(-1) with a determination coefficient of 0.9996. Bifonazole underwent a photocatalytic degradation process under the experimental conditions used. Comparative studies showed that combination of TiO2 /ZnO (1:1 w/w) was a more effective catalyst than TiO2 or ZnO with a degradation rate of up to 67.57% after 24 h of irradiation. Further, kinetic analyses indicated that the photocatalytic degradation of Bifonazole in the mixture of TiO2 /ZnO can be described by a pseudo-first order reaction. Statistical comparison clearly indicated that the presence of TiO2 /ZnO also affected the stability of Bifonazole from a cream preparation after 15 h of UVA exposure (p < 0.05). Ten photodegradation products of Bifonazole were identified for the first time and their plausible fragmentation pathways, derived from MS/MS data, were proposed. The main pathway in the photocatalytic transformation of Bifonazole in the presence of ZnO or/and TiO2 involves hydroxylation of the methanetriyl group and/or adjacent phenyl rings and cleavage of the imidazole moiety.
Crystallization of bifonazole and acetaminophen within the matrix of semicrystalline, PEO-PPO-PEO triblock copolymers.[Pubmed:25569586]
Mol Pharm. 2015 Feb 2;12(2):590-9.
The morphology and microstructure of crystalline drug/polymer solid dispersions could influence their physical stability and dissolution performance. In this study, the drug crystallization mechanism within PEG, PPG, and poloxamer matrix was investigated, and the resultant microstructure of various solid dispersions of acetaminophen (ACM) and Bifonazole (BFZ) in the aforementioned polymers was characterized by differential scanning calorimetry (DSC), polarized optical microscopy (POM), and wide/small-angle X-ray diffraction (WAXD/SAXS). With a stronger molecular interaction with the PEG segments, ACM decreased the crystallization onset temperature and crystallinity of PEG and poloxamers much more than BFZ. The stronger molecular interaction and better miscibility between ACM and PEG also induced a more defective lamellar structure in the ACM solid dispersions compared with that in the BFZ systems, as revealed by DSC and SAXS investigation. Observed under polarized optical microscopy, PEG, PPG, and poloxamer could all significantly improve the crystallization rate of ACM and BFZ, because of the largely reduced Tg of the solid dispersions by these low Tg polymers. Moreover, when the drug loading was below 60%, crystallization of BFZ in PEG or poloxamer occurred preferably along the radial direction of PEG spherulite, rather than the perpendicular direction, which was attributed to the geometric restriction of well-ordered polymer lamellar structure in the BFZ solid dispersions. Similar phenomena were not observed in the ACM solid dispersions regardless of the drug loading, presumably because ACM could diffuse freely across the perpendicular direction of the PEG spherulite, through the well-connected interlamellar or interfibrillar spaces produced by the defective PEG lamellar structure. The different drug-polymer interaction also caused a difference in the microstructure of polymer crystal, as well as a difference in drug distribution within the polymer matrix, which then synergistically facilitated a "confined crystallization" process to reduce the drug crystallite size below 100 nm.
The mechanism of bifonazole-induced [Ca(2+)]i rises and non-Ca(2+)-triggered cell death in PC3 human prostate cancer cells.[Pubmed:24849495]
J Recept Signal Transduct Res. 2014 Dec;34(6):493-9.
Bifonazole is an antifungal drug widely used for treating skin diseases. The effect of Bifonazole on physiology of cancer cells is unclear. The effect of Bifonazole on cytosolic free Ca(2+) concentrations ([Ca(2+)]i) and viability in PC3 human prostate cancer cells was explored. The Ca(2+)-sensitive fluorescent dye, fura-2, was applied to measure [Ca(2+)]i. Bifonazole at concentrations of 5-30 microM induced a [Ca(2+)]i rise in a concentration-dependent manner. The response was reduced by 50% by removing extracellular Ca(2+). Bifonazole-evoked [Ca(2+)]i rise was not altered by nifedipine, econazole, SK&F96365 and protein kinase C activator, but was inhibited by 75% by GF109203X, a protein kinase C inhibitor. In Ca(2+)-free medium, treatment with the endoplasmic reticulum Ca(2+) pump inhibitor 2,5-di-tert-butylhydroquinone (BHQ) nearly abolished Bifonazole-evoked [Ca(2+)]i rise. Conversely, treatment with Bifonazole abolished BHQ-evoked [Ca(2+)]i rise. Inhibition of phospholipase C with U73122 abolished Bifonazole-induced [Ca(2+)]i rise. At 30-100 microM, Bifonazole decreased cell viability concentration-dependently, which was not reversed by chelating cytosolic Ca(2+) with 1,2-bis(2-aminophenoxy)ethane-N,N,N'',N'-tetraacetic acid/acetoxy methyl. Annexin V/propidium iodide staining data suggest that Bifonazole (30-100 microM) induced apoptosis concentration-dependently. Together, in PC3 human prostate cancer cells, Bifonazole induced [Ca(2+)]i rises by inducing phospholipase C- and protein kinase C-dependent Ca(2+) release from the endoplasmic reticulum and Ca(2+) influx via non-store-operated pathways. Bifonazole induced cell death that might involve apoptosis.


