P1075Potent Kir6 (KATP) channel opener CAS# 60559-98-0 |
- USP7-USP47 inhibitor
Catalog No.:BCC4113
CAS No.:1247825-37-1
- NSC 632839 hydrochloride
Catalog No.:BCC2088
CAS No.:157654-67-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 60559-98-0 | SDF | Download SDF |
| PubChem ID | 43345 | Appearance | Powder |
| Formula | C12H17N5 | M.Wt | 231.3 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 50 mM in ethanol and to 100 mM in DMSO | ||
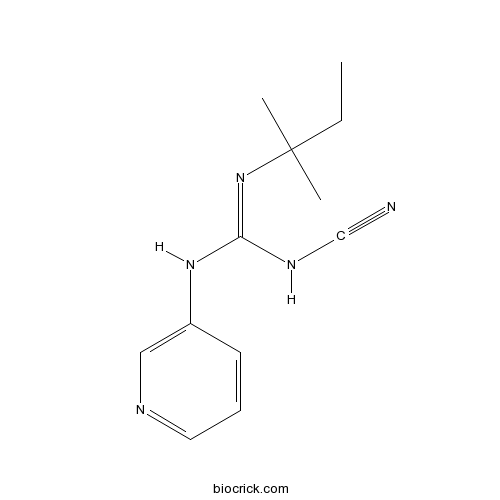
| Chemical Name | 1-cyano-2-(2-methylbutan-2-yl)-3-pyridin-3-ylguanidine | ||
| SMILES | CCC(C)(C)N=C(NC#N)NC1=CN=CC=C1 | ||
| Standard InChIKey | HKZNADVVGXKQDL-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C12H17N5/c1-4-12(2,3)17-11(15-9-13)16-10-6-5-7-14-8-10/h5-8H,4H2,1-3H3,(H2,15,16,17) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent Kir6 (KATP) channel opener (EC50 for relaxation of rat aorta = 7.5 nM). Binds to SUR2A and SUR2B with high affinity (Kd values are 17 and 3 nM respectively). |

P1075 Dilution Calculator

P1075 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.3234 mL | 21.6169 mL | 43.2339 mL | 86.4678 mL | 108.0847 mL |
| 5 mM | 0.8647 mL | 4.3234 mL | 8.6468 mL | 17.2936 mL | 21.6169 mL |
| 10 mM | 0.4323 mL | 2.1617 mL | 4.3234 mL | 8.6468 mL | 10.8085 mL |
| 50 mM | 0.0865 mL | 0.4323 mL | 0.8647 mL | 1.7294 mL | 2.1617 mL |
| 100 mM | 0.0432 mL | 0.2162 mL | 0.4323 mL | 0.8647 mL | 1.0808 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 1-Acetyltagitinin A
Catalog No.:BCN4119
CAS No.:60547-63-9
- Gomisin D
Catalog No.:BCN2268
CAS No.:60546-10-3
- Olsalazine Sodium
Catalog No.:BCC3829
CAS No.:6054-98-4
- Braylin
Catalog No.:BCN4118
CAS No.:6054-10-0
- Methyloleoside
Catalog No.:BCN8079
CAS No.:60539-23-3
- H-D-Thr-OMe.HCl
Catalog No.:BCC2675
CAS No.:60538-15-0
- Zimelidine dihydrochloride
Catalog No.:BCC7173
CAS No.:60525-15-7
- 5,6-Dihydropyridin-2(1H)-one
Catalog No.:BCN4013
CAS No.:6052-73-9
- Serpentinic acid
Catalog No.:BCN4616
CAS No.:605-14-1
- Pifithrin-β
Catalog No.:BCC5503
CAS No.:60477-34-1
- Cytisine Hydrochloride
Catalog No.:BCN8133
CAS No.:6047-01-4
- Cauloside F
Catalog No.:BCN3848
CAS No.:60451-47-0
- DCEBIO
Catalog No.:BCC7060
CAS No.:60563-36-2
- Tirandamycin B
Catalog No.:BCN1862
CAS No.:60587-14-6
- Pamabrom
Catalog No.:BCC1835
CAS No.:606-04-2
- 2,4'-Dihydroxybenzophenone
Catalog No.:BCN3358
CAS No.:606-12-2
- Toyocamycin
Catalog No.:BCC8047
CAS No.:606-58-6
- Cinnabarinic acid
Catalog No.:BCC7865
CAS No.:606-59-7
- Homopterocarpin
Catalog No.:BCN4615
CAS No.:606-91-7
- MK-0773
Catalog No.:BCC1754
CAS No.:606101-58-0
- Momor-cerebroside I
Catalog No.:BCN4120
CAS No.:606125-07-9
- AZD6244 (Selumetinib)
Catalog No.:BCC3624
CAS No.:606143-52-6
- MEK162 (ARRY-162, ARRY-438162)
Catalog No.:BCC1148
CAS No.:606143-89-9
- Bifonazole
Catalog No.:BCC4766
CAS No.:60628-96-8
Different potassium channels are involved in relaxation of rat renal artery induced by P1075.[Pubmed:22225832]
Basic Clin Pharmacol Toxicol. 2012 Jul;111(1):24-30.
The ATP-sensitive K(+) channels opener (K(ATP)CO), P1075 [N-cyano-N'-(1,1-dimethylpropyl)-N''-3-pyridylguanidine], has been shown to cause relaxation of various isolated animal and human blood vessels by opening of vascular smooth muscle ATP-sensitive K(+) (K(ATP)) channels. In addition to the well-known effect on the opening of K(ATP) channels, it has been reported that vasorelaxation induced by some of the K(ATP)COs includes some other K(+) channel subtypes. Given that there is still no information on other types of K(+) channels possibly involved in the mechanism of relaxation induced by P1075, this study was designed to examine the effects of P1075 on the rat renal artery with endothelium and with denuded endothelium and to define the contribution of different K(+) channel subtypes in the P1075 action on this blood vessel. Our results show that P1075 induced a concentration-dependent relaxation of rat renal artery rings pre-contracted by phenylephrine. Glibenclamide, a selective K(ATP) channels inhibitor, partly antagonized the relaxation of rat renal artery induced by P1075. Tetraethylammonium (TEA), a non-selective inhibitor of Ca(2+)-activated K(+) channels, as well as iberiotoxin, a most selective blocker of large-conductance Ca(2+) -activated K(+) (BK(Ca)) channels, did not abolish the effect of P1075 on rat renal artery. In contrast, a non-selective blocker of voltage-gated K(+) (K(V)) channels, 4-aminopyridine (4-AP), as well as margatoxin, a potent inhibitor of K(V)1.3 channels, caused partial inhibition of the P1075-induced relaxation of rat renal artery. In addition, in this study, P1075 relaxed contractions induced by 20 mM K(+) , but had no effect on contractions induced by 80 mM K(+). Our results showed that P1075 induced strong endothelium-independent relaxation of rat renal artery. It seems that K(ATP), 4-AP- and margatoxin-sensitive K(+) channels located in vascular smooth muscle mediated the relaxation of rat renal artery induced by P1075.
Interaction of a novel dihydropyridine K+ channel opener, A-312110, with recombinant sulphonylurea receptors and KATP channels: comparison with the cyanoguanidine P1075.[Pubmed:15023854]
Br J Pharmacol. 2004 Apr;141(7):1098-105.
1. ATP-sensitive K(+) channels (K(ATP) channels) are composed of pore-forming subunits (Kir6.x) and of regulatory subunits, the sulphonylurea receptors (SURx). Synthetic openers of K(ATP) channels form a chemically heterogeneous class of compounds that are of interest in several therapeutic areas. We have investigated the interaction of a novel dihydropyridine opener, A-312110 ((9R)-9-(4-fluoro-3-iodophenyl)-2,3,5,9-tetrahydro-4H-pyrano[3,4-b]thieno [2,3-e]pyridin-8(7H)-one-1,1-dioxide), with SURs and Kir6/SUR channels in comparison to the cyanoguanidine opener P1075. 2. In the presence of 1 mM MgATP, A-312110 bound to SUR2A (the SUR in cardiac and skeletal muscle) and to SUR2B (smooth muscle) with K(i) values of 14 and 18 nM; the corresponding values for P1075 were 16 and 9 nM, respectively. Decreasing the MgATP concentration reduced the affinity of A312110 binding to SUR2A significantly more than that to SUR2B; for P1075, the converse was true. At SUR1 (pancreatic beta-cell), both openers showed little binding up to 100 microM. 3. In the presence of MgATP, both openers inhibited [(3)H]glibenclamide binding to the SUR2 subtypes in a biphasic manner. In the absence of MgATP, the high-affinity component of the inhibition curves was absent. 4. In inside-out patches, the two openers activated the Kir6.2/SUR2A and Kir6.2/SUR2B channels with similar potency (approximately 50 nm). Both were almost 2 x more efficacious in opening the Kir6.2/SUR2B than the Kir6.2/SUR2A channel. 5. The results show that the novel dihydropyridine A-312110 is a potent K(ATP) channel opener with binding and channel-opening properties similar to those of P1075.
Alteration of binding sites for [3H]P1075 and [3H]glibenclamide in renovascular hypertensive rat aorta.[Pubmed:15659117]
Acta Pharmacol Sin. 2005 Jan;26(1):69-76.
AIM: The alterations of the binding sites for ATP-sensitive K+ channel (K(ATP)) openers and blockers in aortic strips were investigated in hypertensive rats. METHODS: Radioligand binding techniques were used to compare the specific binding properties of [3H]P1075 and [3H]glibenclamide (Gli) in normotensive (NWR) and reno-vascular hypertensive rat (RVHR) aortic strips. RESULTS: The KD values of [3H]P1075 binding were increased by 1.5-fold, while the Bmax values were unchanged in RVHR. The IC50 values of P1075 and pinacidil (Pin) for displacing the [3H]P1075 binding in RVHR were increased by 1.8- and 1.7-fold, respectively. The kinetic processes of association and dissociation of [3H]P1075 binding were slower in RVHR. Glibenclamide pretreatment slowed down the kinetic processes of the association and dissociation of [3H]P1075 binding in NWR, but failed to alter the kinetic processes of [3H]P1075 binding in RVHR. The IC50 values of Gli for displacing the [3H]Gli binding at high-affinity sites were increased by 3-fold, while those at low-affinity sites remained to be unchanged in RVHR. The kinetic processes of association of [3H]Gli binding were decreased and those of the dissociation were accelerated in RVHR. The treatment with Pin slowed down the association kinetic processes but accelerated the process of the dissociation of [3H]Gli binding in NWR, but did not alter the kinetics of [3H]Gli binding in RVHR. CONCLUSION: The affinity of binding sites for [3H]P1075 and of high-affinity binding sites for [3H]Gli are decreased, and the negative allosteric interactions between the two binding sites are impaired in RVHR aorta.
The effects of potassium channel opener P1075 on the human saphenous vein and human internal mammary artery.[Pubmed:21346595]
J Cardiovasc Pharmacol. 2011 Jun;57(6):648-55.
Because adrenergic contractions can contribute to the development of life-threatening spasm of coronary artery bypass graft, this study was performed to investigate the effect of adenosine 3-phosphate (ATP)-sensitive K channel (KATP) opener P1075 on contractions of isolated human saphenous vein (HSV) and human internal mammary artery (HIMA). Phasic contractions were evoked by electric field stimulation (20 Hz) and noradrenaline. The sustained contractions were evoked by phenylephrine. The presence of pore-forming Kir6.1 and Kir6.2 subunits of the KATP channels in the HIMA and only Kir6.2 in the HSV was confirmed immunomorphologically. P1075 inhibited in the HSV only, the electrical field stimulation contractions more strongly than noradrenaline contractions. In addition, the phenylephrine contractions of HSV were more sensitive to P1075 in comparison to those of HIMA. Glibenclamide, a KATP channel blocker antagonized the vasodilatation produced by P1075 in both grafts differently, because its effect was more prominent on the P1075-induced inhibition of contractions of HSV than of HIMA. We conclude that P1075 has a vasorelaxant effect and inhibited adrenergic contractions of the tested grafts. This effect is graft and vasoconstrictor selective and seems to be mediated by Kir6.1- and/or Kir6.2-containing KATP channels. Thus, P1075 can be considered as a potential drug in the prevention of graft spasm.
New windows on the mechanism of action of K(ATP) channel openers.[Pubmed:11121575]
Trends Pharmacol Sci. 2000 Nov;21(11):439-45.
K(ATP) channel openers are a diverse group of drugs with a wide range of potential therapeutic uses. Their molecular targets, the K(ATP) channels, exhibit tissue-specific responses because they possess different types of regulatory sulfonylurea receptor subunits. It is well recognized that complex interactions occur between K(ATP) channel openers and nucleotides, but the cloning of the K(ATP) channel has introduced a new dimension to the study of these events and has furthered our understanding of the molecular basis of the action of K(ATP) channel openers.
Pharmacological and molecular analysis of ATP-sensitive K(+) channels in the pig and human detrusor.[Pubmed:10988346]
Eur J Pharmacol. 2000 Jul 21;400(2-3):287-95.
The pharmacological and molecular properties of ATP-sensitive K(+) channels present in pig detrusor smooth muscle were investigated. In isolated pig detrusor strips, ATP-sensitive K(+) channel openers inhibited contractions elicited by low frequency field-stimulation in a concentration-dependent manner. The inhibitory effects of P1075 [N-cyano-N'-(1,1-dimethylpropyl)-N"-3-pyridylguanidine] were attenuated by glyburide with a pA(2) value of 7.38 (slope=1.08). The potency of the inhibitory effects of the K(+) channel openers on the field-stimulated contractions correlated well with those evoked by the muscarinic receptor agonist, carbachol (r=0.93) and furthermore, to relaxation of the pre-contracted (25 mM potassium chloride, KCl) human detrusor (r=0.95). Reverse transcriptase polymerase chain reaction (RT-PCR) analysis showed the presence of mRNA for sulfonylurea receptors SUR1 and SUR2B in both pig and human detrusor. Considering the similarities in the molecular and pharmacological profile of ATP-sensitive K(+) channels between the pig and the human detrusor, it is concluded that the pig detrusor may serve as a suitable in vitro model for the evaluation of novel K(+) channel openers with potential use in urological disorders in humans.
Stoichiometry of potassium channel opener action.[Pubmed:10570067]
Mol Pharmacol. 1999 Dec;56(6):1370-3.
Potassium channel openers (KCOs; e.g., P1075, pinacidil) exert their effects on excitable cells by opening ATP-sensitive potassium channels. These channels are heteromultimers composed with a 4:4 stoichiometry of an inwardly rectifying K(+) channel subunit plus a regulatory subunit comprising the receptor sites for hypoglycemic sulfonylureas and KCOs (a sulfonylurea receptor). To elucidate stoichiometry of KCO action, we analyzed P1075 sensitivity of channels coassembled from sulfonylurea receptor isoforms with high or low P1075 affinity. Concentration activation curves for cDNA ratios of 1:1 or 1:10 resembled those for channel opening resulting from interaction with a single site, whereas models for activation requiring occupation of two, three, or four sites were incongruous. We conclude KCO-induced channel activation to be mediated by interaction with a single binding site per tetradimeric complex.
Tissue and species variation in the vascular receptor binding of 3H-P1075, a potent KATP opener vasodilator.[Pubmed:8996204]
J Pharmacol Exp Ther. 1997 Jan;280(1):255-60.
A high-affinity receptor site for 3H-P1075 previously observed in rat aorta has been proposed to mediate the vasorelaxation effects of P1075 and other ATP-sensitive K+ channel (KATP) openers. We tested this hypothesis by correlating the receptor binding of 3H-P1075 with its vasorelaxation effects in several isolated vascular preparations from three species: rat, rabbit and dog. In rat aorta and mesenteric artery, 3H-P1075 (1-5 nM) showed high amounts of specific binding (5-10 fmol/mg tissue), which was 48 to 79% of total binding. In contrast, little (< or = 17%) to no specific binding of 3H-P1075 (1-5 nM) was observed in dog coronary artery, dog mesenteric artery or rabbit mesenteric artery. However, all vascular preparations studied relaxed with P1075 (1-100 nM), showing maximal relaxations at 30 to 100 nM. The P1075 relaxation EC50 values in rat aorta, rabbit mesenteric artery and dog coronary artery ranged from 7.5 to 24.1 nM depending on the level of contractile activation. Thus, the pharmacological effect of P1075 could be correlated with the presence of specific receptor binding sites only in rat vascular preparations. These data show that there are significant differences in the characteristics of the proposed specific receptor site for 3H-P1075 in different vascular preparations from different species, and they raise questions regarding the pharmacological significance of this KATP opener binding site. Until such questions are resolved, it appears that the study of functional significance of this receptor site as well as further biochemical characterization of this receptor site may necessitate the use of only the rat vascular preparations.


