Pifithrin-βCAS# 60477-34-1 |
- Dihydroberberine
Catalog No.:BCN2573
CAS No.:483-15-8
- Sesamolin
Catalog No.:BCN1289
CAS No.:526-07-8
- Carnosol
Catalog No.:BCN1055
CAS No.:5957-80-2
- Harpagide
Catalog No.:BCN4996
CAS No.:6926-08-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 60477-34-1 | SDF | Download SDF |
| PubChem ID | 443278 | Appearance | Powder |
| Formula | C16H16N2S | M.Wt | 268.38 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Cyclic Pifithrin-α | ||
| Solubility | Soluble in DMSO | ||
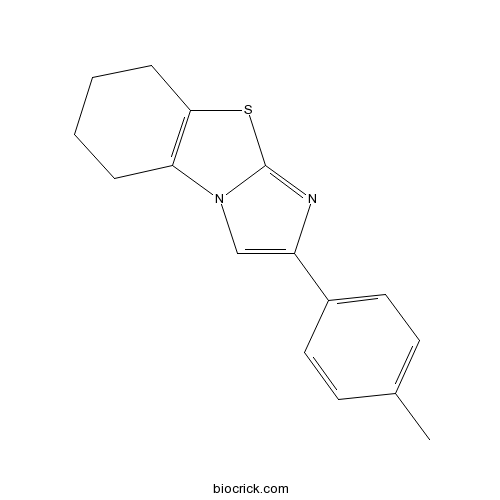
| Chemical Name | 2-(4-methylphenyl)-5,6,7,8-tetrahydroimidazo[2,1-b][1,3]benzothiazole | ||
| SMILES | CC1=CC=C(C=C1)C2=CN3C4=C(CCCC4)SC3=N2 | ||
| Standard InChIKey | IMUKUMUNZJILCG-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C16H16N2S/c1-11-6-8-12(9-7-11)13-10-18-14-4-2-3-5-15(14)19-16(18)17-13/h6-10H,2-5H2,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Pifithrin-β Dilution Calculator

Pifithrin-β Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.7261 mL | 18.6303 mL | 37.2606 mL | 74.5212 mL | 93.1515 mL |
| 5 mM | 0.7452 mL | 3.7261 mL | 7.4521 mL | 14.9042 mL | 18.6303 mL |
| 10 mM | 0.3726 mL | 1.863 mL | 3.7261 mL | 7.4521 mL | 9.3152 mL |
| 50 mM | 0.0745 mL | 0.3726 mL | 0.7452 mL | 1.4904 mL | 1.863 mL |
| 100 mM | 0.0373 mL | 0.1863 mL | 0.3726 mL | 0.7452 mL | 0.9315 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Pifithrin-β is a potent p53 inhibitor with an IC50 of 23 μM.
In Vitro:Pifithrin-α, an inhibitor of the p53 protein, is regarded as a lead compound for cancer and neurodegenerative disease therapy. Pifithrin-α is very unstable in culture medium and rapidly converts to its condensation product pifithrin-β, the N-acetyl derivative[2]. After 24 h, the viability assay shows that the pretreatments with 1 and 10 μM pifithrin-β exerts neuroprotective effects[3].
References:
[1]. Christodoulou MS, et al. Synthesis and biological evaluation of imidazolo[2,1-b]benzothiazole derivatives, as potential p53 inhibitors. Bioorg Med Chem. 2011 Mar 1;19(5):1649-57.
[2]. Fernández-Cruz ML, et al. Biological and chemical studies on aryl hydrocarbon receptor induction by the p53 inhibitor pifithrin-α and its condensation product pifithrin-β. Life Sci. 2011 Apr 25;88(17-18):774-83.
[3]. Da Pozzo E, et al. p53 functional inhibitors behaving like pifithrin-β counteract the Alzheimer peptide non-β-amyloid component effects in human SH-SY5Y cells. ACS Chem Neurosci. 2014 May 21;5(5):390-9.
- Cytisine Hydrochloride
Catalog No.:BCN8133
CAS No.:6047-01-4
- Cauloside F
Catalog No.:BCN3848
CAS No.:60451-47-0
- Neopetasitenine
Catalog No.:BCN2114
CAS No.:60409-51-0
- Cucurbitacin A
Catalog No.:BCN2468
CAS No.:6040-19-3
- Tombozine
Catalog No.:BCN4117
CAS No.:604-99-9
- Narcissoside
Catalog No.:BCN1263
CAS No.:604-80-8
- 7,8-Benzoflavone
Catalog No.:BCN6538
CAS No.:604-59-1
- Doronine
Catalog No.:BCN2106
CAS No.:60367-00-2
- 6,7,8-Trimethoxycoumarin
Catalog No.:BCN4113
CAS No.:6035-49-0
- Leucovorin Calcium
Catalog No.:BCC1198
CAS No.:6035-45-6
- 9-O-Feruloyllariciresinol
Catalog No.:BCN4112
CAS No.:60337-67-9
- LY2090314
Catalog No.:BCC1717
CAS No.:603288-22-8
- Serpentinic acid
Catalog No.:BCN4616
CAS No.:605-14-1
- 5,6-Dihydropyridin-2(1H)-one
Catalog No.:BCN4013
CAS No.:6052-73-9
- Zimelidine dihydrochloride
Catalog No.:BCC7173
CAS No.:60525-15-7
- H-D-Thr-OMe.HCl
Catalog No.:BCC2675
CAS No.:60538-15-0
- Methyloleoside
Catalog No.:BCN8079
CAS No.:60539-23-3
- Braylin
Catalog No.:BCN4118
CAS No.:6054-10-0
- Olsalazine Sodium
Catalog No.:BCC3829
CAS No.:6054-98-4
- Gomisin D
Catalog No.:BCN2268
CAS No.:60546-10-3
- 1-Acetyltagitinin A
Catalog No.:BCN4119
CAS No.:60547-63-9
- P1075
Catalog No.:BCC7027
CAS No.:60559-98-0
- DCEBIO
Catalog No.:BCC7060
CAS No.:60563-36-2
- Tirandamycin B
Catalog No.:BCN1862
CAS No.:60587-14-6
p53 functional inhibitors behaving like pifithrin-beta counteract the Alzheimer peptide non-beta-amyloid component effects in human SH-SY5Y cells.[Pubmed:24646317]
ACS Chem Neurosci. 2014 May 21;5(5):390-9.
Alzheimer's disease (AD) develops from a complex setting of genetic and biochemical alterations, including an increased level of p53 in the brain. Here, the robust and specific activation of p53 by the fibrillar non-beta-amyloid component (NAC) of AD was demonstrated in human neuroblastoma SH-SY5Y cells. For the first time, the increase in the level of p53 target gene transcription, the cell cycle arrest, and the induction of apoptosis elicited by NAC were evidenced. These effects were counterbalanced by pifithrin-beta, a small molecule interfering with the p53 functions. Using the structure of a pifithrin-beta analogue as a reference, a pharmacophore-based virtual screening of the ZINC database was performed. Among the resulting hits, 20 druglike heterocyclic compounds were selected and evaluated for their neuroprotective activity against fibrillar NAC in the human SH-SY5Y cellular model. Three compounds exhibited neuroprotective effects. In particular, 2-(4-methoxyphenyl)-7-methyl-7H-pyrazolo[4,3-e][1,2,4]triazolo[1,5-c]pyrimidine resulted in a promising lead compound for further development of anti-AD agents in terms of neuroprotection, reducing the rate of NAC-induced cell death with an activity higher than that of pifithrin-beta, as a result of a more effective functional inhibition of p53 target gene transcription.
Biological and chemical studies on aryl hydrocarbon receptor induction by the p53 inhibitor pifithrin-alpha and its condensation product pifithrin-beta.[Pubmed:21362431]
Life Sci. 2011 Apr 25;88(17-18):774-83.
AIMS: Pifithrin alpha (PFTalpha), an inhibitor of the p53 protein, is regarded as a lead compound for cancer and neurodegenerative disease therapy. There is some evidence that this compound activates the aryl hydrocarbon receptor (AhR) in a complete independent way of the p53 inhibition and that it is easily converted to its condensation product pifithrin beta (PFTbeta). The aim of this study was to explore the ability of PFTalpha and of PFTbeta to induce a variety of AhR mediated processes. MAIN METHODS: Computational analysis using quantum chemical calculations and chemical analysis have been used to study the conformation of the compounds as well as the cyclization reaction. The AhR mediated processes of these compounds have been studied in a rainbow trout cell line (RTG-2) and in a rat hepatoma cell line (H4IIE). KEY FINDINGS: PFTalpha molecule could not take a planar conformation required for AhR activation whereas PFTbeta showed a conformation similar to those of the prototypical AhR ligand beta-naphthoflavone. In both cell lines, PFTalpha and PFTbeta provoked different responses related with AhR activation. However, when cyclization of PFTalpha to PFTbeta was hampered by acetylation of the exocyclic nitrogen, all these responses were not observed. These results lead to the conclusion that the activation of the AhR is probably caused by PFTbeta instead of PFTalpha. SIGNIFICANCE: Since PFTalpha is a promising compound for the development of new pharmaceuticals inhibiting p53, the chemical instability of this compound as well as the capacity of its transformation product should be taken into account.


