Cytisine HydrochlorideCAS# 6047-01-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 6047-01-4 | SDF | Download SDF |
| PubChem ID | 20056518 | Appearance | Powder |
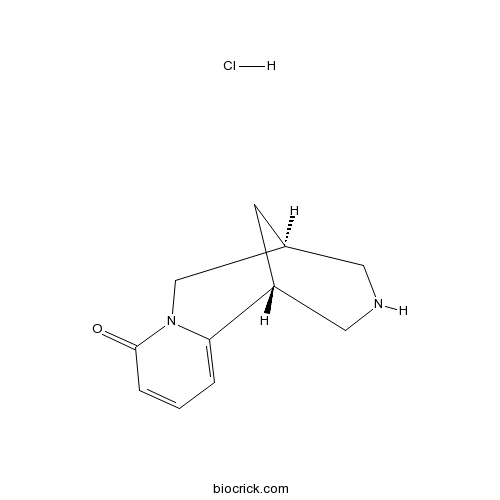
| Formula | C11H15ClN2O | M.Wt | 226.70 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (1R,9S)-7,11-diazatricyclo[7.3.1.02,7]trideca-2,4-dien-6-one;hydrochloride | ||
| SMILES | C1C2CNCC1C3=CC=CC(=O)N3C2.Cl | ||
| Standard InChIKey | NNYPHEBIGWBCNH-OULXEKPRSA-N | ||
| Standard InChI | InChI=1S/C11H14N2O.ClH/c14-11-3-1-2-10-9-4-8(5-12-6-9)7-13(10)11;/h1-3,8-9,12H,4-7H2;1H/t8-,9+;/m0./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Cytisine hydrochloride has toxicity. |

Cytisine Hydrochloride Dilution Calculator

Cytisine Hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.4111 mL | 22.0556 mL | 44.1112 mL | 88.2223 mL | 110.2779 mL |
| 5 mM | 0.8822 mL | 4.4111 mL | 8.8222 mL | 17.6445 mL | 22.0556 mL |
| 10 mM | 0.4411 mL | 2.2056 mL | 4.4111 mL | 8.8222 mL | 11.0278 mL |
| 50 mM | 0.0882 mL | 0.4411 mL | 0.8822 mL | 1.7644 mL | 2.2056 mL |
| 100 mM | 0.0441 mL | 0.2206 mL | 0.4411 mL | 0.8822 mL | 1.1028 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Cauloside F
Catalog No.:BCN3848
CAS No.:60451-47-0
- Neopetasitenine
Catalog No.:BCN2114
CAS No.:60409-51-0
- Cucurbitacin A
Catalog No.:BCN2468
CAS No.:6040-19-3
- Tombozine
Catalog No.:BCN4117
CAS No.:604-99-9
- Narcissoside
Catalog No.:BCN1263
CAS No.:604-80-8
- 7,8-Benzoflavone
Catalog No.:BCN6538
CAS No.:604-59-1
- Doronine
Catalog No.:BCN2106
CAS No.:60367-00-2
- 6,7,8-Trimethoxycoumarin
Catalog No.:BCN4113
CAS No.:6035-49-0
- Leucovorin Calcium
Catalog No.:BCC1198
CAS No.:6035-45-6
- 9-O-Feruloyllariciresinol
Catalog No.:BCN4112
CAS No.:60337-67-9
- LY2090314
Catalog No.:BCC1717
CAS No.:603288-22-8
- GSK-3 inhibitor 1
Catalog No.:BCC4126
CAS No.:603272-51-1
- Pifithrin-β
Catalog No.:BCC5503
CAS No.:60477-34-1
- Serpentinic acid
Catalog No.:BCN4616
CAS No.:605-14-1
- 5,6-Dihydropyridin-2(1H)-one
Catalog No.:BCN4013
CAS No.:6052-73-9
- Zimelidine dihydrochloride
Catalog No.:BCC7173
CAS No.:60525-15-7
- H-D-Thr-OMe.HCl
Catalog No.:BCC2675
CAS No.:60538-15-0
- Methyloleoside
Catalog No.:BCN8079
CAS No.:60539-23-3
- Braylin
Catalog No.:BCN4118
CAS No.:6054-10-0
- Olsalazine Sodium
Catalog No.:BCC3829
CAS No.:6054-98-4
- Gomisin D
Catalog No.:BCN2268
CAS No.:60546-10-3
- 1-Acetyltagitinin A
Catalog No.:BCN4119
CAS No.:60547-63-9
- P1075
Catalog No.:BCC7027
CAS No.:60559-98-0
- DCEBIO
Catalog No.:BCC7060
CAS No.:60563-36-2
Some studies on cytisine and its methylated derivatives.[Pubmed:4387392]
Br J Pharmacol. 1969 Jan;35(1):161-74.
1. In mice Cytisine Hydrochloride is less toxic intravenously than nicotine hydrogen tartrate, but more toxic by intraperitoneal or oral administration. Compared with cytisine, caulophylline hydrogen iodide is one-fifth to one-tenth as toxic and caulophylline methiodide is less than one-thirtieth as toxic.2. The surprising low oral toxicity of cytisine and nicotine may be ascribed to the method of administration; if the drug is placed directly in the stomach there is no possibility of absorption through buccal mucous membranes.3. The peripheral effects of nicotine, cytisine and caulophylline are similar, though on some preparations those of nicotine last longer. In most tests cytisine is active in doses from a quarter to three-quarters of those of nicotine, caulophylline in doses from 10 to 20 times those of cytisine. Caulophylline methiodide is virtually inactive.4. Cytisine and caulophylline may differ from nicotine in their central effects.5. Cytisine and caulophylline are active as the cations. The pKa of cytisine is 7.92 and that of caulophylline is 7.04; the difference accounts, in part, for the weaker activity of caulophylline. The caulophylline ion is generally one-sixth to one-third as active as the cytisine ion.6. The introduction of the second methyl group to form the quaternary salt does not appear to cause a dramatic change in the conformation of the molecule. Caulophylline methiodide appears to be feebly active because it has feeble affinity.


