DihydromolluginCAS# 60657-93-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 60657-93-4 | SDF | Download SDF |
| PubChem ID | 10779560 | Appearance | Powder |
| Formula | C17H18O4 | M.Wt | 286.32 |
| Type of Compound | Quinones | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
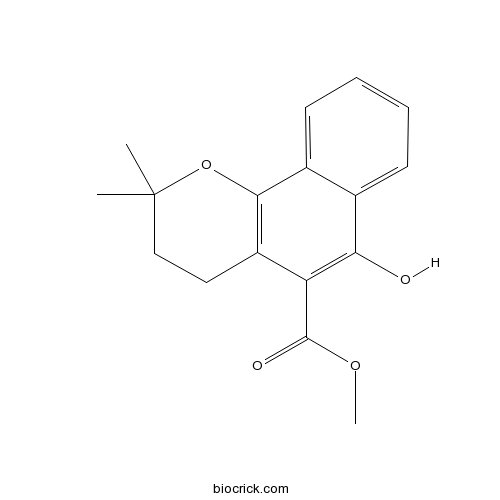
| Chemical Name | methyl 6-hydroxy-2,2-dimethyl-3,4-dihydrobenzo[h]chromene-5-carboxylate | ||
| SMILES | CC1(CCC2=C(O1)C3=CC=CC=C3C(=C2C(=O)OC)O)C | ||
| Standard InChIKey | AKPYYGWMASCNAF-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C17H18O4/c1-17(2)9-8-12-13(16(19)20-3)14(18)10-6-4-5-7-11(10)15(12)21-17/h4-7,18H,8-9H2,1-3H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Structure Identification | Phytochemistry, 28(12):3465-3468.Anthraquinones and naphthohydroquinones from Rubia cordifolia.[Reference: WebLink]
Journal of the Chinese Chemical Society, 2001,48(1):77-79.Synthesis of Naturally Occurring Rubilactone, Mollugin, and Dihydromollugin of Rubia cordifolia.[Reference: WebLink]
Rubilactone (1), Dihydromollugin (2), and mollugin (3) are naturally occurring products found in Rubia cordifolia, which is a famous Chinese herb with anti tumor, viral inhibition and other activities. Synthetic studies were carried out in these naphthoic acid esters starting from 1,4-dihydroxy-2-naphthoic acid.
|

Dihydromollugin Dilution Calculator

Dihydromollugin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.4926 mL | 17.463 mL | 34.926 mL | 69.8519 mL | 87.3149 mL |
| 5 mM | 0.6985 mL | 3.4926 mL | 6.9852 mL | 13.9704 mL | 17.463 mL |
| 10 mM | 0.3493 mL | 1.7463 mL | 3.4926 mL | 6.9852 mL | 8.7315 mL |
| 50 mM | 0.0699 mL | 0.3493 mL | 0.6985 mL | 1.397 mL | 1.7463 mL |
| 100 mM | 0.0349 mL | 0.1746 mL | 0.3493 mL | 0.6985 mL | 0.8731 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Danaidone
Catalog No.:BCN1966
CAS No.:6064-85-3
- Bifonazole
Catalog No.:BCC4766
CAS No.:60628-96-8
- MEK162 (ARRY-162, ARRY-438162)
Catalog No.:BCC1148
CAS No.:606143-89-9
- AZD6244 (Selumetinib)
Catalog No.:BCC3624
CAS No.:606143-52-6
- Momor-cerebroside I
Catalog No.:BCN4120
CAS No.:606125-07-9
- MK-0773
Catalog No.:BCC1754
CAS No.:606101-58-0
- Homopterocarpin
Catalog No.:BCN4615
CAS No.:606-91-7
- Cinnabarinic acid
Catalog No.:BCC7865
CAS No.:606-59-7
- Toyocamycin
Catalog No.:BCC8047
CAS No.:606-58-6
- 2,4'-Dihydroxybenzophenone
Catalog No.:BCN3358
CAS No.:606-12-2
- Pamabrom
Catalog No.:BCC1835
CAS No.:606-04-2
- Tirandamycin B
Catalog No.:BCN1862
CAS No.:60587-14-6
- 3-n-Butylphthalide
Catalog No.:BCN2381
CAS No.:6066-49-5
- HOSu
Catalog No.:BCC2845
CAS No.:6066-82-6
- Isopicropodophyllone
Catalog No.:BCN8316
CAS No.:60660-50-6
- 3-Hydroxy-1,5-diphenyl-1-pentanone
Catalog No.:BCN3536
CAS No.:60669-64-9
- FC 131
Catalog No.:BCC7917
CAS No.:606968-52-9
- Sesamin
Catalog No.:BCN4123
CAS No.:607-80-7
- Myristicin
Catalog No.:BCN2730
CAS No.:607-91-0
- Physoperuvine
Catalog No.:BCN1402
CAS No.:60723-27-5
- SB 772077B dihydrochloride
Catalog No.:BCC6116
CAS No.:607373-46-6
- AZ 10606120 dihydrochloride
Catalog No.:BCC6005
CAS No.:607378-18-7
- Canthin-6-one N-oxide
Catalog No.:BCN2992
CAS No.:60755-87-5
- 2-Hydroxy-1,8-cineole
Catalog No.:BCN4121
CAS No.:60761-00-4
Efficient synthesis of cis- and trans-3,4-dihydroxy-3,4-dihydromollugin.[Pubmed:18412388]
J Org Chem. 2008 May 16;73(10):3867-74.
An efficient synthesis of naturally occurring compounds isolated from Pentas longiflora, cis-3,4-dihydroxy-3,4-Dihydromollugin 2, and trans-3,4-dihydroxy-3,4-Dihydromollugin 3 is described. The O-protected mollugins were dihydroxylated using OsO4 to achieve the corresponding cis-dihydroxy derivatives in excellent yield. The synthesis of trans-3,4-dihydroxy-3,4-Dihydromollugin was achieved using Oxone in good yield. A mechanism for the formation of cis-3,4-dihydroxymollugin acetonide from the reaction of mollugin with Oxone is proposed.
New pyranonaphthoquinone and pyranonaphthohydroquinone from the roots of Pentas longiflora.[Pubmed:12350172]
J Nat Prod. 2002 Sep;65(9):1377-9.
Several quinone type compounds were isolated from the hexane, dichloromethane, and ethyl acetate extracts of the roots of Pentas longiflora. The hexane extract afforded two new compounds, [(3alpha,3'alpha,4beta,4'beta)-3,3']-dimethoxy-cis-[4,4'-bis(3,4,5,10-tetrahydro- 1H-naphtho[2,3-c]pyran)]-5,5',10,10'-tetraone (1) and cis-3,4-dihydroxy-3,4-Dihydromollugin (2), together with six known compounds, namely, pentalongin, mollugin, trans-3,4-dihydroxy-3,4-Dihydromollugin, methyl-2,3-epoxy-3-prenyl-1,4-naphthoquinone-2-carboxylate, tectoquinone, and 3-hydroxymollugin. From the dichloromethane extract were isolated the three known compounds 3-methoxymollugin, methyl-3-prenyl-1,4-naphthoquinone-2-carboxylate, and scopoletin, while the ethyl acetate extract afforded the known 2-methoxy-3-methylanthraquinone.
[Studies on naphthoic acid esters from the roots of Rubia cordifolia L].[Pubmed:1442042]
Yao Xue Xue Bao. 1992;27(4):279-82.
Four naphthoic acid esters including a new compound were isolated from the roots of Rubia cordifolia L. The new one was named as rubilactone and its structure was elucidated as 3'-carbomethoxy-4'-hydroxy-naphtho[1',2'-2,3] pyran-6-one (I) based on the physicochemical properties and spectrometric analyses (UV, IR, MS, 1HNMR and 13CNMR). The other three were 3'-carbomethoxy-4'-hydroxy-naphtho [1',2'-2,3] furan (II), Dihydromollugin (III) and 3-carbomethoxy-2-(3'-hydroxy)isopentyl-1,4-naphthohydroquinone-1-O -beta-D-glucoside (IV).


