Cnidium monnieri
Cnidium monnieri
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Cnidium monnieri
- Cat.No. Product Name CAS Number COA
-
BCN4881
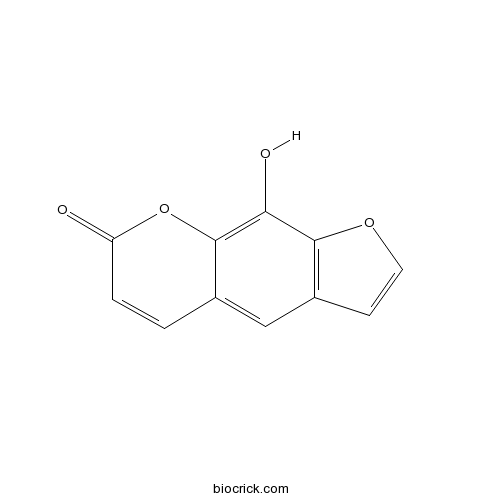
Xanthotoxol2009-24-7
Instructions
-
BCN5205
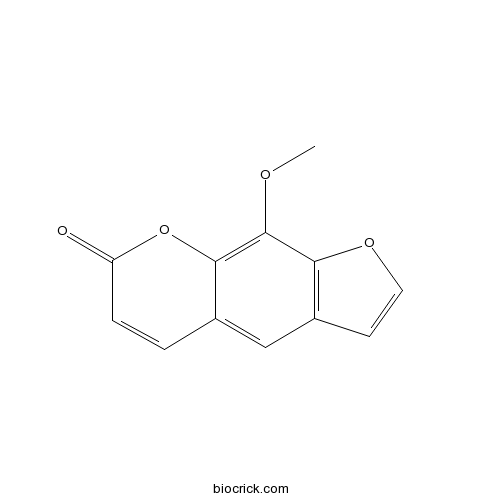
Xanthotoxin298-81-7
Instructions
-
BCN5568
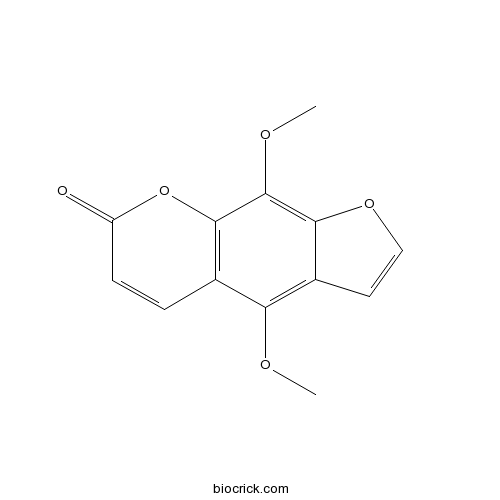
Isopimpinellin482-27-9
Instructions
-
BCN5897
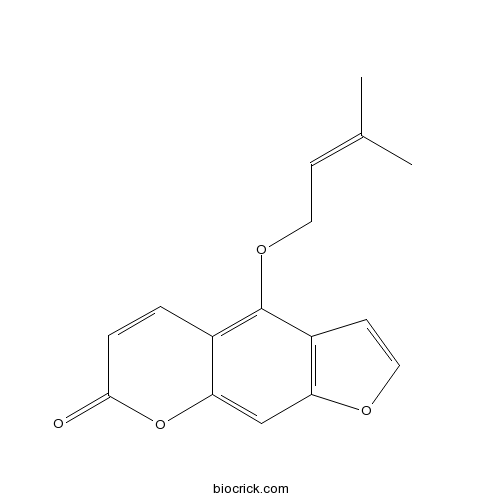
Isoimperatorin482-45-1
Instructions
-
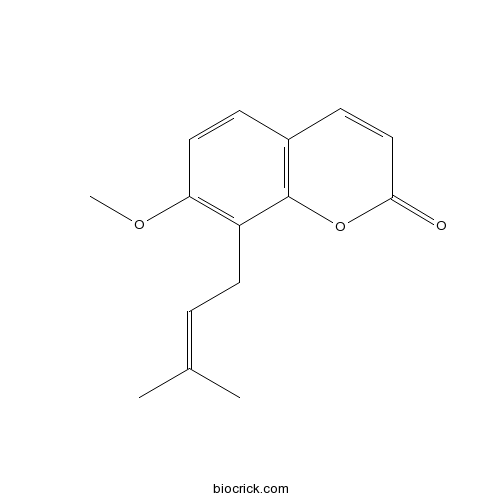
BCN5581
Osthol484-12-8
Instructions
-
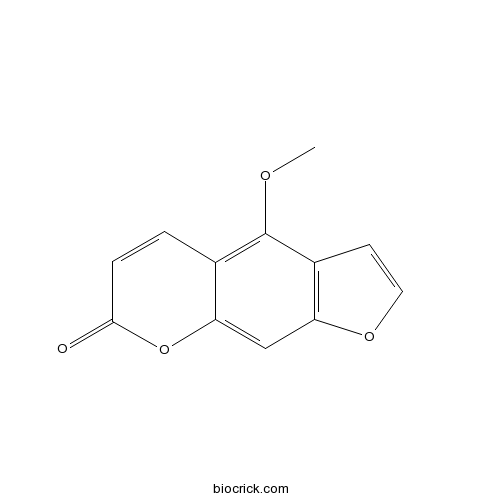
BCN5582
Bergapten484-20-8
Instructions
-
BCN8334
Thymine65-71-4
Instructions

Anti-pruritic effect of natural coumarin osthole through selective inhibition of thermosensitive TRPV3 channel in the skin.[Pubmed: 30108138]
Coumarin osthole is a dominant bioactive ingredient of natural Cnidium monnieri plant commonly used as traditional Chinese herbal medicines for therapies and treatments including antipruritus and antidermatitis. However, the molecular mechanism underlying the action of osthole remains unclear. In this study we report that osthole exerts antipruritic effect through selective inhibition of Ca2+-permeable and thermosensitive TRPV3 cation channels that are primarily expressed in the keratinocytes of the skin. Coumarin osthole was identified as an inhibitor of TRPV3 channels transiently expressed in HEK293 cells in the calcium fluorescent FlexStation assay. Inhibition of TRPV3 current by osthole and its selectivity were further confirmed by whole-cell patch clamp recordings of TRPV3 expressing HEK293 cells and mouse primary cultured keratinocytes. Behavioral evaluation demonstrated that inhibition of TRPV3 by osthole or silencing by knockout of TRPV3 gene significantly reduced the scratching induced by either acetone-ether-water (AEW) or histamine in localized rostral neck skin in mice. Taken together, our findings provide a molecular basis for use of natural coumarin osthole from C. monnieri plant in antipruritic or skin care therapy, thus establishing a significant role of TRPV3 channel in chronic itch signaling or acute histamine-dependent itch sensation.
Osthole inhibits gastric cancer cell proliferation through regulation of PI3K/AKT.[Pubmed: 29590128]
Osthole is an active compound isolated from Chinese herb Cnidium monnieri (L.) Cusson, and had been reported to possess antitumor effect. However, the effect of osthole on the gastric cancer cells has not been investigated. In this study, the effects of osthole on the proliferation of human gastric cancer cells were tested. The data showed that osthole treatment significantly inhibited the proliferation of gastric cancer cells and resulted in the cell cycle arrest at G2/M phase in a dose-dependent manner. Western-blot study showed that the expression of cyclin B1 and cdc2 was markedly reduced by osthole. Moreover, expression of PI3K and pAKT was also significantly suppressed, and the results indicated that the inhibition of pAKT, cyclin B1, and cdc2 levels by osthole was notably enhanced by a PI3K inhibitor. These results demonstrate that osthole could inhibit gastric cancer cells proliferation via induction of cell cycle arrest at G2/M phase by the reduction of PI3K/AKT.
Ethanol extract from Cnidium monnieri (L.) Cusson induces cell cycle arrest and apoptosis via regulation of the p53‑independent pathway in HepG2 and Hep3B hepatocellular carcinoma cells.[Pubmed: 29207130]
Cnidium monnieri (L.) Cusson is a frequently used traditional Chinese medicine that treats gynecological diseases and carbuncles. However, the mechanism of action of C. monnieri remains to be fully elucidated. The present study examined the cell cycle arrest and apoptotic effects resulting from ethanol extract of C. monnieri (CME) in HepG2 (wild‑type p53) and Hep3B (p53‑null) hepatocellular carcinoma cells. An MTT assay was used to confirm the anti‑proliferative effect of CME. The cells were stained with Hoechst 33342 or propidium iodide. It was demonstrated that proliferation of HepG2 cells was suppressed by CME. Cell cycle arrest occurred in the G1 phase following treatment with CME and the number of apoptotic bodies was increased. The expression levels of cell cycle‑associated proteins, including protein kinase B (Akt), glycogen synthase kinase‑3β (GSK‑3β), p53, cyclin E and cyclin‑dependent kinase 2 (CDK2) were determined by western blot analysis. The protein levels of phosphorylated (p)‑Akt, p‑GSK‑3β, p‑MDM2 and cyclin E were decreased, whereas the protein levels of p53, p21 and p‑CDK2 (Thr14/Tyr15) were increased following treatment with CME. Furthermore, treatment or co‑treatment with LY294002 (phosphoinositide‑3‑kinase/Akt inhibitor) or Pifithrin‑α (p53 inhibitor) with CME resulted in CME‑induced G1 arrest which occurred through the p53‑independent signaling pathway in hepatocellular carcinoma cells. In conclusion, CME induces G1 arrest and apoptosis via the Akt/GSK‑3β signaling pathway which is regulated by MDM2‑induced degradation of p21, rather than p53.


