IsopimpinellinCAS# 482-27-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 482-27-9 | SDF | Download SDF |
| PubChem ID | 68079 | Appearance | White-yellowish powder |
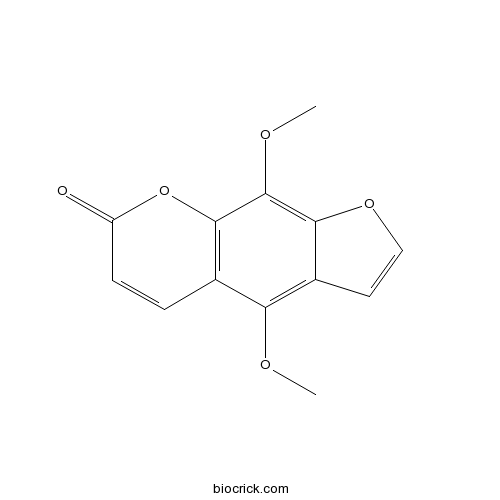
| Formula | C13H10O5 | M.Wt | 246.2 |
| Type of Compound | Coumarins | Storage | Desiccate at -20°C |
| Synonyms | 5,8-Dimethoxypsoralen | ||
| Solubility | Soluble in acetone and chloroform; practically insoluble in water | ||
| Chemical Name | 4,9-dimethoxyfuro[3,2-g]chromen-7-one | ||
| SMILES | COC1=C2C=COC2=C(C3=C1C=CC(=O)O3)OC | ||
| Standard InChIKey | DFMAXQKDIGCMTL-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C13H10O5/c1-15-10-7-3-4-9(14)18-12(7)13(16-2)11-8(10)5-6-17-11/h3-6H,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Isopimpinellin has chemopreventive effects, it effectively inhibits mouse COH activity (IC50 values 19-40 microM).Isopimpinellin is a new inhibitor against the Leishmania APRT enzyme. |
| Targets | BACE | Antifection |
| In vitro | Isopimpinellin is not phototoxic in a chick skin assay.[Pubmed: 8881335]Photochem Photobiol. 1996 Mar;63(3):306-7.
Isopimpinellin is not phototoxic to viruses and cells.[Pubmed: 3628561 ]Planta Med. 1987 Jun;53(3):306-7.Isopimpinellin is not phototoxic to viruses and cells. Redetermination and comparative structural study of isopimpinellin: A new inhibitor against the Leishmania APRT enzyme[Reference: WebLink]Acta Crystallographica, 2003, 59(10):o1506–8.The title compound (Isopimpinellin,alternative name 5,8-dimethoxypsoralen), C13H10O5, is a natural product extracted from Adiscanthus fusciflorus (Rutaceae). |
| In vivo | Oral administration of the citrus coumarin, isopimpinellin, blocks DNA adduct formation and skin tumor initiation by 7,12-dimethylbenz[a]anthracene in SENCAR mice.[Pubmed: 12376476]Carcinogenesis. 2002 Oct;23(10):1667-75.The current study was designed to evaluate the effects of oral administration of the citrus coumarin, Isopimpinellin, on skin tumor initiation by topically applied benzo[a]pyrene (B[a]P) and 7,12-dimethylbenz[a]anthracene (DMBA). |
| Structure Identification | J Oleo Sci. 2011;60(11):575-8.Microbial transformation of Isopimpinellin by Glomerella cingulata.[Pubmed: 22027023]
|

Isopimpinellin Dilution Calculator

Isopimpinellin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.0617 mL | 20.3087 mL | 40.6174 mL | 81.2348 mL | 101.5435 mL |
| 5 mM | 0.8123 mL | 4.0617 mL | 8.1235 mL | 16.247 mL | 20.3087 mL |
| 10 mM | 0.4062 mL | 2.0309 mL | 4.0617 mL | 8.1235 mL | 10.1543 mL |
| 50 mM | 0.0812 mL | 0.4062 mL | 0.8123 mL | 1.6247 mL | 2.0309 mL |
| 100 mM | 0.0406 mL | 0.2031 mL | 0.4062 mL | 0.8123 mL | 1.0154 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Byakangelicin 2'-O-Isovalerate
Catalog No.:BCC8899
CAS No.:108006-56-0
- Homoferreirin
Catalog No.:BCN4765
CAS No.:482-01-9
- Estriol 3-sulfate
Catalog No.:BCN2236
CAS No.:481-95-8
- Chrysophanol
Catalog No.:BCN5567
CAS No.:481-74-3
- Citreorosein
Catalog No.:BCN5566
CAS No.:481-73-2
- Aloeemodin
Catalog No.:BCN5565
CAS No.:481-72-1
- Tangeretin
Catalog No.:BCN2386
CAS No.:481-53-8
- Cepharanthine
Catalog No.:BCN5393
CAS No.:481-49-2
- Ginkgetin
Catalog No.:BCN2319
CAS No.:481-46-9
- Plumbagin
Catalog No.:BCN2586
CAS No.:481-42-5
- Juglone
Catalog No.:BCN2639
CAS No.:481-39-0
- Ecgonine
Catalog No.:BCN1907
CAS No.:481-37-8
- Isoquercitrin
Catalog No.:BCN5569
CAS No.:482-35-9
- Hyperoside
Catalog No.:BCN5570
CAS No.:482-36-0
- Kaempferitrin
Catalog No.:BCN5572
CAS No.:482-38-2
- Afzelin
Catalog No.:BCN5573
CAS No.:482-39-3
- Imperatorin
Catalog No.:BCN5574
CAS No.:482-44-0
- Isoimperatorin
Catalog No.:BCN5897
CAS No.:482-45-1
- Isobergapten
Catalog No.:BCN2377
CAS No.:482-48-4
- Osajin
Catalog No.:BCN4789
CAS No.:482-53-1
- Sarpagine
Catalog No.:BCN5575
CAS No.:482-68-8
- Nordalbergin
Catalog No.:BCC8344
CAS No.:482-82-6
- Dalbergin
Catalog No.:BCN7452
CAS No.:482-83-7
- Indigo
Catalog No.:BCN1091
CAS No.:482-89-3
Oral administration of the citrus coumarin, isopimpinellin, blocks DNA adduct formation and skin tumor initiation by 7,12-dimethylbenz[a]anthracene in SENCAR mice.[Pubmed:12376476]
Carcinogenesis. 2002 Oct;23(10):1667-75.
The current study was designed to evaluate the effects of oral administration of the citrus coumarin, Isopimpinellin, on skin tumor initiation by topically applied benzo[a]pyrene (B[a]P) and 7,12-dimethylbenz[a]anthracene (DMBA). To evaluate the effects of orally administered Isopimpinellin on skin tumor initiation by B[a]P and DMBA, its effects on DNA adduct formation were first evaluated. Female SENCAR mice were pre-treated twice with corn oil, or Isopimpinellin (70 mg/kg body wt per os) at 24 h and 2 h prior to topical treatment with B[a]P or DMBA. Another citrus coumarin, imperatorin, was also included in these experiments for comparison. Orally administered Isopimpinellin and imperatorin significantly inhibited B[a]P-DNA adduct formation by 37 and 26%, respectively. Imperatorin also blocked DMBA-DNA adduct formation by 43%. In a second dose-response study, orally administered Isopimpinellin (35, 70 and 150 mg/kg) blocked DMBA-DNA adduct formation by 23, 56 and 69%, respectively. For the tumor study, mice were pretreated orally with corn oil or Isopimpinellin at 24 and 2 h prior to initiation with DMBA, and 2 weeks later promotion began with 12-O-tetradecanoylphorbol-13-acetate (TPA). Isopimpinellin significantly reduced the mean number of papillomas per mouse by 49, 73 and 78% compared to corn oil controls at 30, 70 and 150 mg/kg body wt, respectively. Orally administered Isopimpinellin also significantly reduced the percentage of mice with papillomas at the highest dose tested (150 mg/kg). The effectiveness of Isopimpinellin given topically over a broad dose range against DMBA tumor initiation was also evaluated for comparison. As part of this study, several parameters of systemic toxicity were evaluated following oral dosing with Isopimpinellin and imperatorin. Mice were treated orally with corn oil, Isopimpinellin or imperatorin (35, 70 and 150 mg/kg body wt per os) once daily for four consecutive days, killed at 24 h after the last dose, and livers, lungs, and kidneys evaluated histologically. In addition, urinary parameters of nephrotoxicity, blood parameters of liver and kidney function, and thrombin clotting time were assayed. No significant changes in blood clotting, or renal or hepatic function were observed. There was, however, a significant increase in liver wt accompanied by cytoplasmic vacuolation of hepatocytes. There were no histopathological changes in lungs or kidneys. Overall, these data indicate that Isopimpinellin (and imperatorin) have chemopreventive effects when administered orally on skin tumor initiation by DMBA.
Isopimpinellin is not phototoxic in a chick skin assay.[Pubmed:8881335]
Photochem Photobiol. 1996 Mar;63(3):306-7.
Synthetic Isopimpinellin (5,8-dimethoxypsoralen), confirmed to contain as impurities only trace quantities at most of psoralen, bergapten (5-methoxypsoralen) and xanthotoxin (8-methoxypsoralen), is not phototoxic when tested in a chick skin bioassay system. These findings are at variance with earlier studies showing Isopimpinellin to be phototoxic against chick skin and support the conclusion that Isopimpinellin is photobiologically inactive. As recently proposed by others, the several reports of Isopimpinellin photoactivity are most likely attributable to contamination by small amounts of highly active psoralens such as bergapten or xanthotoxin.
Microbial transformation of Isopimpinellin by Glomerella cingulata.[Pubmed:22027023]
J Oleo Sci. 2011;60(11):575-8.
Microbial transformation studies conducted on Isopimpinellin (1) by the fungus Glomerella cingulata have revealed that 1 was metabolized to give the corresponding reduced acid, 5,8-dimethoxy-6,7-furano-hydrocoumaric acid (2). The structure of metabolite 2 was elucidated by high-resolution mass spectrometry (HR-MS), extensive NMR techniques, including (1)H NMR, (13)C NMR, (1)H-(1)H correlation spectroscopy (COSY), heteronuclear multiple quantum coherence (HMQC) and heteonuclear multiple bond coherence (HMBC). The biotransformed product 2 showed weak a in vitro beta-secretase (BACE1) inhibitory effect.


