IndigoCAS# 482-89-3 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 482-89-3 | SDF | Download SDF |
| PubChem ID | 5354391 | Appearance | Purple powder |
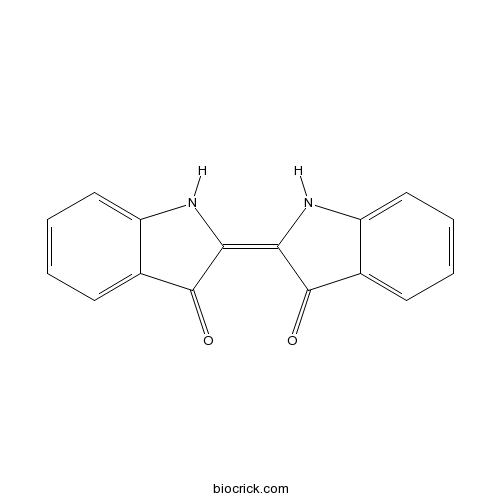
| Formula | C16H10N2O2 | M.Wt | 262.26 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Synonyms | Indigo | ||
| Solubility | Very slightly soluble in water | ||
| Chemical Name | (2Z)-2-(3-oxo-1H-indol-2-ylidene)-1H-indol-3-one | ||
| SMILES | C1=CC=C2C(=C1)C(=O)C(=C3C(=O)C4=CC=CC=C4N3)N2 | ||
| Standard InChIKey | COHYTHOBJLSHDF-YPKPFQOOSA-N | ||
| Standard InChI | InChI=1S/C16H10N2O2/c19-15-9-5-1-3-7-11(9)17-13(15)14-16(20)10-6-2-4-8-12(10)18-14/h1-8,17-18H/b14-13- | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Indigo and indirubin are potent aryl hydrocarbon receptor ligands present in human urine, the endogenous levels and potencies of indirubin and indigo are such that they activate aryl hydrocarbon receptor (AhR) -mediated signaling mechanisms in vivo . Indigo participates in isolating oxygenase genes, indigo carmine angiography provides visual information on foot perfusion, using indigo dye and synergistic halide additives can inhibit mild steel corrosion in sulphuric acid. |
| Targets | Src | Antifection | AhR |
| In vitro | Isolation of oxygenase genes for indigo-forming activity from an artificially polluted soil metagenome by functional screening using Pseudomonas putida strains as hosts.[Pubmed: 25573469]Appl Microbiol Biotechnol. 2015 May;99(10):4453-70.Metagenomes contain the DNA from many microorganisms, both culturable and non-culturable, and are a potential resource of novel genes.
Indirubin and Indigo Are Potent Aryl Hydrocarbon Receptor Ligands Present in Human Urine.[Pubmed: 11425848]J. Biol. Chem., 2001, 276(34):31475-8.Aryl hydrocarbon receptor (AhR) is a ligand-activated transcription factor that regulates genes involved in xenobiotic metabolism, cellular proliferation, and differentiation. Numerous xenobiotic and biological compounds are known to interact with AhR, but it remains an orphan receptor, because its physiological ligand is unknown.
|
| In vivo | Use of Indigo Carmine Angiography to Qualitatively Assess Adequate Distal Perfusion After Endovascular Revascularization in Critical Limb Ischemia.[Pubmed: 25887729]J Endovasc Ther. 2015 Apr 17.To report a novel technique to visualize the efficacy of revascularization in critical limb ischemia patients with ischemic foot ulcers.
|
| Kinase Assay | Inhibition of mild steel corrosion in sulphuric acid using indigo dye and synergistic halide additives[Reference: WebLink]Mater. Chem. Phys., 2004, 84(2-3): 363-8.Gravimetric method was used to study the inhibitory properties of Indigo dye during corrosion of mild steel in aerated sulphuric acid solutions at 30-50°C. The effect of addition of halide salts KCl, KBr and KI was also investigated. The corrosion rates in all systems studied increased with rise in temperature. The inhibition efficiency of Indigo dye increased with concentration and synergistically increased on addition of halide salts. Temperature studies revealed increased inhibition efficiency at higher temperatures, which is suggestive of chemisorption mechanism. The inhibitor adsorption characteristics were approximated by Frumkins isotherm and Flory-Huggins isotherm. Activation energy for Fe dissolution in sulphuric acid was observed to reduce from 54.6kJmol-1 in the uninhibited system to 34.9kJmol-1 in the inhibited system. |

Indigo Dilution Calculator

Indigo Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.813 mL | 19.065 mL | 38.1301 mL | 76.2602 mL | 95.3252 mL |
| 5 mM | 0.7626 mL | 3.813 mL | 7.626 mL | 15.252 mL | 19.065 mL |
| 10 mM | 0.3813 mL | 1.9065 mL | 3.813 mL | 7.626 mL | 9.5325 mL |
| 50 mM | 0.0763 mL | 0.3813 mL | 0.7626 mL | 1.5252 mL | 1.9065 mL |
| 100 mM | 0.0381 mL | 0.1907 mL | 0.3813 mL | 0.7626 mL | 0.9533 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Dalbergin
Catalog No.:BCN7452
CAS No.:482-83-7
- Nordalbergin
Catalog No.:BCC8344
CAS No.:482-82-6
- Sarpagine
Catalog No.:BCN5575
CAS No.:482-68-8
- Osajin
Catalog No.:BCN4789
CAS No.:482-53-1
- Isobergapten
Catalog No.:BCN2377
CAS No.:482-48-4
- Isoimperatorin
Catalog No.:BCN5897
CAS No.:482-45-1
- Imperatorin
Catalog No.:BCN5574
CAS No.:482-44-0
- Afzelin
Catalog No.:BCN5573
CAS No.:482-39-3
- Kaempferitrin
Catalog No.:BCN5572
CAS No.:482-38-2
- Hyperoside
Catalog No.:BCN5570
CAS No.:482-36-0
- Isoquercitrin
Catalog No.:BCN5569
CAS No.:482-35-9
- Isopimpinellin
Catalog No.:BCN5568
CAS No.:482-27-9
- Aricine
Catalog No.:BCN5576
CAS No.:482-91-7
- RG 108
Catalog No.:BCC1134
CAS No.:48208-26-0
- Tetrahydroamentoflavone
Catalog No.:BCN5571
CAS No.:48236-96-0
- 14-Dehydrobrowniine
Catalog No.:BCN8109
CAS No.:4829-56-5
- Ajmalicine
Catalog No.:BCN5577
CAS No.:483-04-5
- Isorauhimbine
Catalog No.:BCN5578
CAS No.:483-09-0
- 9-Hydroxycalabaxanthone hydrate
Catalog No.:BCC5325
CAS No.:483-14-7
- Dihydroberberine
Catalog No.:BCN2573
CAS No.:483-15-8
- Cephaeline
Catalog No.:BCC8143
CAS No.:483-17-0
- (-)-Isocorypalmine
Catalog No.:BCN2723
CAS No.:483-34-1
- Cheilanthifoline
Catalog No.:BCN7827
CAS No.:483-44-3
- Sphondin
Catalog No.:BCN5579
CAS No.:483-66-9
Indirubin and indigo are potent aryl hydrocarbon receptor ligands present in human urine.[Pubmed:11425848]
J Biol Chem. 2001 Aug 24;276(34):31475-8.
Aryl hydrocarbon receptor (AhR) is a ligand-activated transcription factor that regulates genes involved in xenobiotic metabolism, cellular proliferation, and differentiation. Numerous xenobiotic and biological compounds are known to interact with AhR, but it remains an orphan receptor, because its physiological ligand is unknown. We identified AhR ligands in human urine using a yeast AhR signaling assay and then characterized their properties. Two ligands, indirubin and Indigo, were both present at average concentrations of approximately 0.2 nm in the urine of normal donors. Indirubin was also detected in fetal bovine serum and contributed half of the total AhR ligand activity. The activities of indirubin and Indigo were comparable with or more potent than that of the archetypal ligand, 2,3,7,8-tetrachlorodibenzo-p-dioxin, in yeast AhR activation assays. We suggest that the endogenous levels and potencies of indirubin and Indigo are such that they activate AhR-mediated signaling mechanisms in vivo.
Isolation of oxygenase genes for indigo-forming activity from an artificially polluted soil metagenome by functional screening using Pseudomonas putida strains as hosts.[Pubmed:25573469]
Appl Microbiol Biotechnol. 2015 May;99(10):4453-70.
Metagenomes contain the DNA from many microorganisms, both culturable and non-culturable, and are a potential resource of novel genes. In this study, a 5.2-Gb metagenomic DNA library was constructed from a soil sample (artificially polluted with four aromatic compounds, i.e., biphenyl, phenanthrene, carbazole, and 3-chlorobenzoate) in Escherichia coli by using a broad-host-range cosmid vector. The resultant library was introduced into naphthalene-degrading Pseudomonas putida-derived strains having deficiencies in their naphthalene dioxygenase components, and Indigo-forming clones on the indole-containing agar plates were screened. Cosmids isolated from 29 positive clones were classified by their various properties (original screening hosts, hosts showing Indigo-forming activity, and digestion patterns with restriction enzymes), and six representative cosmids were chosen. Sequencing and in vitro transposon mutagenesis of the six cosmids resulted in the identification of genes encoding putative class B and D flavoprotein monooxygenases, a multicomponent hydroxylase, and a reductase that were responsible for the Indigo-forming activity in the host cells. Among them, the genes encoding the multicomponent hydroxylase were demonstrated to be involved in phenol degradation. Furthermore, two genes encoding ring-cleavage dioxygenases were also found adjacent to the genes responsible for the Indigo formation, and their functions were experimentally confirmed.
Use of indigo carmine angiography to qualitatively assess adequate distal perfusion after endovascular revascularization in critical limb ischemia.[Pubmed:25887729]
J Endovasc Ther. 2015 Jun;22(3):352-5.
PURPOSE: To report a novel technique to visualize the efficacy of revascularization in critical limb ischemia patients with ischemic foot ulcers. TECHNIQUE: An 80-year-old man was admitted with nonhealing ulcers on his left second toe and lateral border of the foot owing to in-stent restenosis of the left popliteal artery. After dilation of the popliteal in-stent restenosis, below-the-knee angiography revealed that the anterior tibial artery (ATA) was occluded, the posterior tibial artery was hypoplastic, and the peroneal artery was enlarged, with 2 plantar arteries. To evaluate the foot circulation before performing additional procedures, a 4-F multipurpose catheter was advanced into the peroneal artery, and 5 mL of Indigo carmine was injected. Immediately, the patient's second toe and lateral border ulcers were dyed blue. We concluded that sufficient blood flow had been obtained to the ulcerated area by balloon angioplasty alone, so the procedure was terminated. The ulcers completely healed at 1 month. CONCLUSION: Indigo carmine angiography provides visual information on foot perfusion, yielding new insights into microcirculation and helping to determine the effectiveness of treatment and procedure endpoint.


