IsobergaptenCAS# 482-48-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 482-48-4 | SDF | Download SDF |
| PubChem ID | 68082 | Appearance | White powder |
| Formula | C12H8O4 | M.Wt | 216.19 |
| Type of Compound | Coumarins | Storage | Desiccate at -20°C |
| Synonyms | 5-Methoxyangelicin | ||
| Solubility | Soluble in chloroform and methanol; insoluble in water | ||
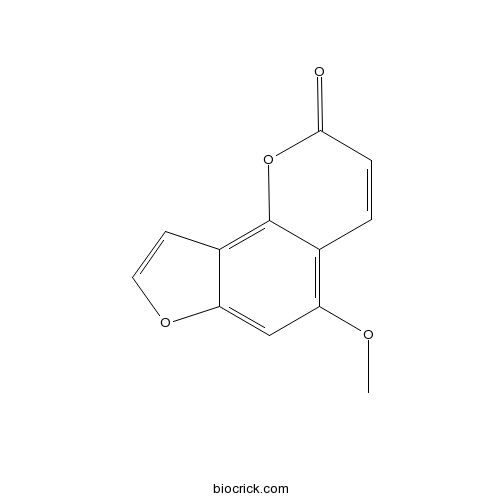
| Chemical Name | 5-methoxyfuro[2,3-h]chromen-2-one | ||
| SMILES | COC1=C2C=CC(=O)OC2=C3C=COC3=C1 | ||
| Standard InChIKey | AJSPSRWWZBBIOR-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C12H8O4/c1-14-9-6-10-8(4-5-15-10)12-7(9)2-3-11(13)16-12/h2-6H,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Isobergapten is a plant growth regulating substance, it is the principal constituents responsible for the antimycobacterial activity of the roots of Heracleum maximum; it may form an important class of natural defensive agents against fungi.Isobergapten shows anti-proliferative activity and causes G2/M arrest at concentrations of 0.05-15.0 μM. |
| Targets | Chk | Antifection | CDC |
| In vitro | The Canadian medicinal plant Heracleum maximum contains antimycobacterial diynes and furanocoumarins.[Pubmed: 23501157]J Ethnopharmacol. 2013 May 2;147(1):232-7.Heracleum maximum is amongst the most commonly used plants by the indigenous peoples of North America. The First Nations of the eastern Canada use infusions of Heracleum maximum roots for the treatment of respiratory ailments including tuberculosis. Previous investigations of extracts derived from the roots of Heracleum maximum have shown it to possess antimycobacterial activity. To isolate and identify antimycobacterial constituents from the roots of Heracleum maximum. Plant growth regulators from Heracleum lanatum.[Reference: WebLink]Phytochemistry, 1982, 21(9):2213-5.The plant growth regulating substances, pimpinellin, isopimpinellin, bergapten, Isobergapten, vaginidiol, sphondin, scopoletin, umbelliferone, ferulic. The fungitoxicities of plant furocoumarins.[Reference: WebLink]Ann. Appl. Biol., 1966, 57(3):501-8.SUMMARYA mixture of the furocoumarins pimpinellin, isopimpinellin, Isobergapten and sphondin isolated from Heracleum sphondylium root was toxic to Gloeosporium limetticola, Botryis cinerea, Sclerotinia fructigena and Stereum purpureum at 200 p.p.m. or less in nutrient medium. The extractive of the leaves of plants that contain furocoumarins suppressed in vivo growth of G. limetticola and B. cinerea at concentrations lower than the contents of the extractive in the leaves. |
| Kinase Assay | Anti-tumor effects of various furocoumarins isolated from the roots, seeds and fruits of Angelica and Cnidium species under ultraviolet A irradiation.[Pubmed: 23649674]J Nat Med. 2014 Jan;68(1):83-94.We examined the effects on cell proliferation of 10 methoxyfurocoumarins and 7 dihydrofurocumarins isolated from Umbelliferae medicinal plants, and their mechanisms of action against B16F10 melanoma cells or in melanin-possessing hairless mice implanted with B16F10 melanoma cells, under UVA irradiation. |

Isobergapten Dilution Calculator

Isobergapten Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.6256 mL | 23.1278 mL | 46.2556 mL | 92.5112 mL | 115.639 mL |
| 5 mM | 0.9251 mL | 4.6256 mL | 9.2511 mL | 18.5022 mL | 23.1278 mL |
| 10 mM | 0.4626 mL | 2.3128 mL | 4.6256 mL | 9.2511 mL | 11.5639 mL |
| 50 mM | 0.0925 mL | 0.4626 mL | 0.9251 mL | 1.8502 mL | 2.3128 mL |
| 100 mM | 0.0463 mL | 0.2313 mL | 0.4626 mL | 0.9251 mL | 1.1564 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Isoimperatorin
Catalog No.:BCN5897
CAS No.:482-45-1
- Imperatorin
Catalog No.:BCN5574
CAS No.:482-44-0
- Afzelin
Catalog No.:BCN5573
CAS No.:482-39-3
- Kaempferitrin
Catalog No.:BCN5572
CAS No.:482-38-2
- Hyperoside
Catalog No.:BCN5570
CAS No.:482-36-0
- Isoquercitrin
Catalog No.:BCN5569
CAS No.:482-35-9
- Isopimpinellin
Catalog No.:BCN5568
CAS No.:482-27-9
- Byakangelicin 2'-O-Isovalerate
Catalog No.:BCC8899
CAS No.:108006-56-0
- Homoferreirin
Catalog No.:BCN4765
CAS No.:482-01-9
- Estriol 3-sulfate
Catalog No.:BCN2236
CAS No.:481-95-8
- Chrysophanol
Catalog No.:BCN5567
CAS No.:481-74-3
- Citreorosein
Catalog No.:BCN5566
CAS No.:481-73-2
- Osajin
Catalog No.:BCN4789
CAS No.:482-53-1
- Sarpagine
Catalog No.:BCN5575
CAS No.:482-68-8
- Nordalbergin
Catalog No.:BCC8344
CAS No.:482-82-6
- Dalbergin
Catalog No.:BCN7452
CAS No.:482-83-7
- Indigo
Catalog No.:BCN1091
CAS No.:482-89-3
- Aricine
Catalog No.:BCN5576
CAS No.:482-91-7
- RG 108
Catalog No.:BCC1134
CAS No.:48208-26-0
- Tetrahydroamentoflavone
Catalog No.:BCN5571
CAS No.:48236-96-0
- 14-Dehydrobrowniine
Catalog No.:BCN8109
CAS No.:4829-56-5
- Ajmalicine
Catalog No.:BCN5577
CAS No.:483-04-5
- Isorauhimbine
Catalog No.:BCN5578
CAS No.:483-09-0
- 9-Hydroxycalabaxanthone hydrate
Catalog No.:BCC5325
CAS No.:483-14-7
Anti-tumor effects of various furocoumarins isolated from the roots, seeds and fruits of Angelica and Cnidium species under ultraviolet A irradiation.[Pubmed:23649674]
J Nat Med. 2014 Jan;68(1):83-94.
We examined the effects on cell proliferation of 10 methoxyfurocoumarins and 7 dihydrofurocumarins isolated from Umbelliferae medicinal plants, and their mechanisms of action against B16F10 melanoma cells or in melanin-possessing hairless mice implanted with B16F10 melanoma cells, under UVA irradiation. Furocoumarins having a methoxy group, such as bergapten (1), xanthotoxin (2), phellopterin (4), byakangelicin (6), neobyakangelicin (8), Isobergapten (9) and sphondin (10), showed anti-proliferative activity and caused G2/M arrest at concentrations of 0.05-15.0 muM. The 7 dihydrofurocoumarins had no effect. UVA plus 1, 2, 4, 6 and sec-O-acetylbyakagelicin (7), having one methoxy group at the C-5 position and a linear-type conformation, reduced tumor growth and final tumor weight in B16F10-bearing mice at 0.5 or 1.0 mg/kg (intraperitoneal injection). UVA plus 1 and 2 increased Chk1 phosphorylation and decreased cdc2 (Thr 161) phosphorylation in the melanoma cells. The anti-tumor actions of UVA plus furocoumarins having a methoxy group might be due to the arrest of the cell cycle at G2/M through an increase in phospho-Chk1 and reduction in phospho-cdc2.
The Canadian medicinal plant Heracleum maximum contains antimycobacterial diynes and furanocoumarins.[Pubmed:23501157]
J Ethnopharmacol. 2013 May 2;147(1):232-7.
ETHNOPHARMACOLOGICAL RELEVANCE: Heracleum maximum is amongst the most commonly used plants by the indigenous peoples of North America. The First Nations of the eastern Canada use infusions of Heracleum maximum roots for the treatment of respiratory ailments including tuberculosis. Previous investigations of extracts derived from the roots of Heracleum maximum have shown it to possess antimycobacterial activity. AIM OF THE STUDY: To isolate and identify antimycobacterial constituents from the roots of Heracleum maximum. MATERIALS AND METHODS: A methanolic extract of Heracleum maximum roots was subjected to bioassay guided fractionation using the microplate resazurin assay (MRA) to assess inhibitory activity against Mycobacterium tuberculosis strain H37Ra. The antimycobacterial constituents were identified by NMR, MS and polarimetry. RESULTS: The polyacetylene (3R,8S)-falcarindiol and the furanocoumarins bergapten, Isobergapten, angelicin, sphondin, pimpinellin, isopimpinellin and 6-isopentenyloxyIsobergapten were isolated from the Heracleum maximum root extract. (3R,8S)-Falcarindiol and 6-isopentenyloxyIsobergapten exhibited MICs of 24 muM and 167 muM and IC50s of 6 muM and 27 muM against Mycobacterium tuberculosis H37Ra respectively. The remaining furanocoumarins bergapten, Isobergapten, angelicin, sphondin, pimpinellin, and isopimpinellin were less active, with MICs of 925, 1850, 2149, 1859, 812 and 1625 muM and IC50s of 125, 344, 350, 351, 389 and 406 muM. CONCLUSIONS: (3R,8S)-Falcarindiol, bergapten, Isobergapten, angelicin, sphondin, pimpinellin, isopimpinellin and 6-isopentenyloxyIsobergapten were identified as the principal constituents responsible for the antimycobacterial activity of the roots of Heracleum maximum. This work supports the ethnopharmacological use of Heracleum maximum by Canadian First Nations and Native American communities as a treatment for infectious diseases, specifically tuberculosis.


