TetrahydroamentoflavoneCAS# 48236-96-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 48236-96-0 | SDF | Download SDF |
| PubChem ID | 326004 | Appearance | Yellow powder |
| Formula | C30H22O10 | M.Wt | 542.5 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
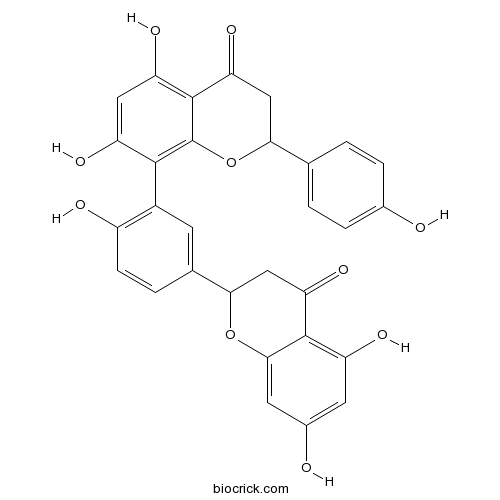
| Chemical Name | 8-[5-(5,7-dihydroxy-4-oxo-2,3-dihydrochromen-2-yl)-2-hydroxyphenyl]-5,7-dihydroxy-2-(4-hydroxyphenyl)-2,3-dihydrochromen-4-one | ||
| SMILES | C1C(OC2=C(C(=CC(=C2C1=O)O)O)C3=C(C=CC(=C3)C4CC(=O)C5=C(C=C(C=C5O4)O)O)O)C6=CC=C(C=C6)O | ||
| Standard InChIKey | ULTQJSQDLWNWTR-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C30H22O10/c31-15-4-1-13(2-5-15)24-12-23(38)29-21(36)10-20(35)27(30(29)40-24)17-7-14(3-6-18(17)33)25-11-22(37)28-19(34)8-16(32)9-26(28)39-25/h1-10,24-25,31-36H,11-12H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. (2S,2″S )-Tetrahydroamentoflavone exerts its antioxidant activity in vitro through metal-chelating, and radical-scavenging, which is via donating a hydrogen atom (H61) and an electron (e). 2. Tetrahydroamentoflavone is a potent XO inhibitor, may be considered as a drug candidate or chemopreventive agent. 3. Tetrahydroamentoflavone has anti-inflammatory effects, it shows in vitro cyclooxygenase (COX) inhibition activity ( an IC(50) value of 29.5 microM (COX-1) and 40.5% inhibition at 100 microg/mL (COX-2)). |
| Targets | COX |

Tetrahydroamentoflavone Dilution Calculator

Tetrahydroamentoflavone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.8433 mL | 9.2166 mL | 18.4332 mL | 36.8664 mL | 46.0829 mL |
| 5 mM | 0.3687 mL | 1.8433 mL | 3.6866 mL | 7.3733 mL | 9.2166 mL |
| 10 mM | 0.1843 mL | 0.9217 mL | 1.8433 mL | 3.6866 mL | 4.6083 mL |
| 50 mM | 0.0369 mL | 0.1843 mL | 0.3687 mL | 0.7373 mL | 0.9217 mL |
| 100 mM | 0.0184 mL | 0.0922 mL | 0.1843 mL | 0.3687 mL | 0.4608 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- RG 108
Catalog No.:BCC1134
CAS No.:48208-26-0
- Aricine
Catalog No.:BCN5576
CAS No.:482-91-7
- Indigo
Catalog No.:BCN1091
CAS No.:482-89-3
- Dalbergin
Catalog No.:BCN7452
CAS No.:482-83-7
- Nordalbergin
Catalog No.:BCC8344
CAS No.:482-82-6
- Sarpagine
Catalog No.:BCN5575
CAS No.:482-68-8
- Osajin
Catalog No.:BCN4789
CAS No.:482-53-1
- Isobergapten
Catalog No.:BCN2377
CAS No.:482-48-4
- Isoimperatorin
Catalog No.:BCN5897
CAS No.:482-45-1
- Imperatorin
Catalog No.:BCN5574
CAS No.:482-44-0
- Afzelin
Catalog No.:BCN5573
CAS No.:482-39-3
- Kaempferitrin
Catalog No.:BCN5572
CAS No.:482-38-2
- 14-Dehydrobrowniine
Catalog No.:BCN8109
CAS No.:4829-56-5
- Ajmalicine
Catalog No.:BCN5577
CAS No.:483-04-5
- Isorauhimbine
Catalog No.:BCN5578
CAS No.:483-09-0
- 9-Hydroxycalabaxanthone hydrate
Catalog No.:BCC5325
CAS No.:483-14-7
- Dihydroberberine
Catalog No.:BCN2573
CAS No.:483-15-8
- Cephaeline
Catalog No.:BCC8143
CAS No.:483-17-0
- (-)-Isocorypalmine
Catalog No.:BCN2723
CAS No.:483-34-1
- Cheilanthifoline
Catalog No.:BCN7827
CAS No.:483-44-3
- Sphondin
Catalog No.:BCN5579
CAS No.:483-66-9
- Toddalolactone
Catalog No.:BCN2393
CAS No.:483-90-9
- Calycanthoside
Catalog No.:BCN5580
CAS No.:483-91-0
- Luvangetin
Catalog No.:BCN7527
CAS No.:483-92-1
Tetrahydroamentoflavone (THA) from Semecarpus anacardium as a potent inhibitor of xanthine oxidase.[Pubmed:20965242]
J Ethnopharmacol. 2011 Feb 16;133(3):1117-20.
ETHNOPHARMACOLOGICAL RELEVANCE: Seed of Semecarpus anacardium L. is widely used in Indian traditional medicine; Ayurveda and Sidha, for treatment of inflammatory disorders and gout. AIM OF THE STUDY: The present study was aimed at isolation of a compound for its potential to inhibit xanthine oxidase (XO), over expression of which lead to inflammation and gout. MATERIALS AND METHODS: Activity guided fractionation of S. anacardium seed was conducted using liquid-liquid partition and preparative HPLC. The fractions were evaluated for their XO inhibition and antioxidant activity. The ethyl acetate fraction with the highest XO activity yielded a biflavonoid compound Tetrahydroamentoflavone (THA). Lineweaver-Burk (LB) plot for the XO inhibition of THA and allopurinol was constructed from the kinetic data. RESULTS: IC(5)(0) values of THA and allopurinol for XO inhibition were 92 and 100 nM respectively and their corresponding values for K(i) were 0.982 and 0.612 muM respectively. CONCLUSION: THA was a potent XO inhibitor which could be considered as a drug candidate or chemopreventive agent, after establishing its pharmacological and clinical evaluation. The study results appear to support the claim of the traditional medicine with respect to the efficacy of S. anacardium seed against inflammation and gout.
A cyclooxygenase (COX) inhibitory biflavonoid from the seeds of Semecarpus anacardium.[Pubmed:15507338]
J Ethnopharmacol. 2004 Dec;95(2-3):209-12.
Semecarpus anacardium Linn., Anacardiaceae, is being most commonly used in India for the treatment of rheumatoid arthritis and other inflammatory disorders. Bioactivity guided fractionation of ethyl acetate extract led to the isolation of major active principle, Tetrahydroamentoflavone (THA), a biflavonoid. The in vitro cyclooxygenase (COX-1) catalyzed prostaglandin biosynthesis assay of THA gave an IC(50) value of 29.5 microM (COX-1) and 40.5% inhibition at 100 microg/mL (COX-2). The in vivo carrageenan induced paw edema assay resulted in dose dependent anti-inflammatory effect of THA and the activity was comparable to that of ibuprofen, one of the well known NSAIDs.


