XanthotoxolCAS# 2009-24-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 2009-24-7 | SDF | Download SDF |
| PubChem ID | 65090 | Appearance | White-yellowish powder |
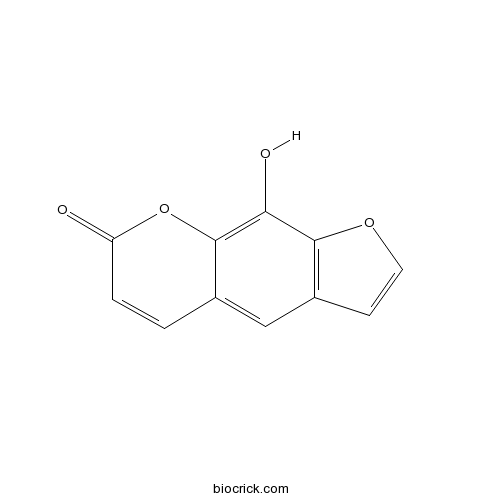
| Formula | C11H6O4 | M.Wt | 202.2 |
| Type of Compound | Coumarins | Storage | Desiccate at -20°C |
| Synonyms | 8-Hydroxypsoralen | ||
| Solubility | DMSO : 123 mg/mL (608.43 mM; Need ultrasonic and warming) | ||
| Chemical Name | 9-hydroxyfuro[3,2-g]chromen-7-one | ||
| SMILES | C1=CC(=O)OC2=C(C3=C(C=CO3)C=C21)O | ||
| Standard InChIKey | JWVYQQGERKEAHW-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C11H6O4/c12-8-2-1-6-5-7-3-4-14-10(7)9(13)11(6)15-8/h1-5,13H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Xanthotoxol shows strong pharmacological activities as anti-inflammatory, antioxidant, cytotoxic, dose-graded sedative, 5-HT antagonistic, and neuroprotective effects.Xanthotoxol also has calcium antagonistic effects, it blocks not only the voltage dependent calcium channel, but also the receptor operated calcium channel in the isolated guinea pig atria. |
| Targets | IL Receptor | TNF-α | NO | NOS | COX | p65 | NF-kB | DNA/RNA Synthesis | 5-HT Receptor |
| In vitro | Cytotoxic effect of xanthotoxol (8-hydroxypsoralen) on TCTC cells in vitro.[Pubmed: 1437852 ]Pol J Pharmacol Pharm. 1992 Jan-Feb;44(1):51-7.The effect of Xanthotoxol (8-hydroxypsoralen) on proliferation of TCTC cells in vitro has been studied. |
| In vivo | Xanthotoxol exerts neuroprotective effects via suppression of the inflammatory response in a rat model of focal cerebral ischemia.[Pubmed: 23619720]Cell Mol Neurobiol. 2013 Jul;33(5):715-22.We previously found that Xanthotoxol, one of the major active ingredients in Cnidium monnieri (L.) Cusson, exerts protective effects in a rat model of focal cerebral ischemia/reperfusion injury by alleviating brain edema, inhibiting the neutrophil infiltration, and decreasing the expression of intercellular adhesion molecule-1 (ICAM-1) and E-selectin. Calcium antagonistic effect of Xanthotoxol on isolated guinea pig atria.[Pubmed: 16104510]Zhong Yao Cai. 2005 Apr;28(4):319-21.To study the mechanism of depressant effect of Xanthotoxol (XT) on contractility in the isolated guinea pig atria.
|
| Kinase Assay | Xanthotoxol (XT) - a potent 5-HT antagonist.[Pubmed: 489098]Indian J Physiol Pharmacol. 1979 Apr-Jun;23(2):142-3.Xanthotoxol (XT) - a potent 5-HT antagonist. |
| Animal Research | Evaluation of xanthotoxol for central nervous system activity.[Pubmed: 1434683]J Ethnopharmacol. 1992 Jun;36(3):239-47.Xanthotoxol (XT), 8-hydroxypsoralen, exhibited dose-graded sedative activity in dogs, cats, rats, mice and hamsters. |
| Structure Identification | Eur J Med Chem. 2010 Jun;45(6):2559-66.Synthesis of methyl-substituted xanthotoxol to clarify prooxidant effect of methyl on radical-induced oxidation of DNA.[Pubmed: 20332056]
|

Xanthotoxol Dilution Calculator

Xanthotoxol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.9456 mL | 24.728 mL | 49.456 mL | 98.912 mL | 123.64 mL |
| 5 mM | 0.9891 mL | 4.9456 mL | 9.8912 mL | 19.7824 mL | 24.728 mL |
| 10 mM | 0.4946 mL | 2.4728 mL | 4.9456 mL | 9.8912 mL | 12.364 mL |
| 50 mM | 0.0989 mL | 0.4946 mL | 0.9891 mL | 1.9782 mL | 2.4728 mL |
| 100 mM | 0.0495 mL | 0.2473 mL | 0.4946 mL | 0.9891 mL | 1.2364 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Epinodosin
Catalog No.:BCN3282
CAS No.:20086-60-6
- Diosbulbin C
Catalog No.:BCN4880
CAS No.:20086-07-1
- Diosbulbin B
Catalog No.:BCN4879
CAS No.:20086-06-0
- Epicurzerenone
Catalog No.:BCN3521
CAS No.:20085-85-2
- Pseudoneolinderane
Catalog No.:BCN8034
CAS No.:20082-45-5
- 16-Nor-15-oxodehydroabietic acid
Catalog No.:BCN3943
CAS No.:200813-31-6
- 7-Hydroxy-PIPAT maleate
Catalog No.:BCC6760
CAS No.:200722-46-9
- (-)-Phyllocladene
Catalog No.:BCN7661
CAS No.:20070-61-5
- Piplartine
Catalog No.:BCN4037
CAS No.:20069-09-4
- Hennadiol
Catalog No.:BCN4679
CAS No.:20065-99-0
- Fmoc-D-Gln(Trt)-OH
Catalog No.:BCC3488
CAS No.:200623-62-7
- Fmoc-D-Gln-OPfp
Catalog No.:BCC3487
CAS No.:200622-33-9
- (D)-(+)-Neopterin
Catalog No.:BCC7960
CAS No.:2009-64-5
- m-3M3FBS
Catalog No.:BCC7209
CAS No.:200933-14-8
- SB 243213 dihydrochloride
Catalog No.:BCC6035
CAS No.:200940-23-4
- Ac-RYYRWK-NH2
Catalog No.:BCC5755
CAS No.:200959-47-3
- Ac-RYYRIK-NH2
Catalog No.:BCC5736
CAS No.:200959-48-4
- Fmoc-Lys(Me)3-OH Chloride
Catalog No.:BCC3267
CAS No.:201004-29-7
- SB-269970
Catalog No.:BCC1927
CAS No.:201038-74-6
- Ravenine
Catalog No.:BCN6666
CAS No.:20105-22-0
- cis-Methylkhellactone
Catalog No.:BCN7690
CAS No.:20107-13-5
- 2-Amino-2'-chloro-5-nitro benzophenone
Catalog No.:BCC8521
CAS No.:2011-66-7
- 4-PPBP maleate
Catalog No.:BCC6723
CAS No.:201216-39-9
- 3-AQC
Catalog No.:BCC6743
CAS No.:201216-42-4
Evaluation of xanthotoxol for central nervous system activity.[Pubmed:1434683]
J Ethnopharmacol. 1992 Jun;36(3):239-47.
Xanthotoxol (XT), 8-hydroxypsoralen, exhibited dose-graded sedative activity in dogs, cats, rats, mice and hamsters. At doses of 5-20 mg/kg intraperitoneally (i.p.) in cats and 3-100 mg/kg orally (p.o.) in dogs, XT blocked predatory mouse/rat killing behavior. In mice, XT (10-300 mg/kg i.p.) exhibited a dose-dependent reduction in locomotor activity but was less potent in this regard than reference diazepam (10-100 mg/kg i.p.). XT in mice (0.1-10.0 mg/kg i.p.) and in hamsters (0.1-10.0 mg/kg p.o.) antagonized amphetamine-induced hypermobility but was less potent than diazepam. XT elevated the electrical threshold in foot-shock-induced fighting behavior in rats. XT (0.1-30.0 mg/kg p.o.) potentiated pentobarbital-induced narcosis in hamsters at otherwise subeffective doses of pentobarbital. Conditioned avoidance responses in rats were not significantly altered with 1-3 mg/kg i.p. and 30-100 mg/kg p.o. doses of XT but 300 mg/kg p.o. blocked both conditioned and unconditioned response. Doses of 100-1000 mg/kg i.p. of XT in mice were used to study 48-h acute toxicity of XT and its LD50 was estimated to be 468 mg/kg. Doses of 10, 40 and 80 mg/kg p.o. were used to study the chronic toxicity of XT in rats for 6 months and no side effects or abnormalities in reproductive activity or endocrine integrity were noted. The F1 generation of rats from 6-month XT-treated parents were free of teratogenic effects.
Synthesis of methyl-substituted xanthotoxol to clarify prooxidant effect of methyl on radical-induced oxidation of DNA.[Pubmed:20332056]
Eur J Med Chem. 2010 Jun;45(6):2559-66.
4-methyl-8-hydroxylpsoralen (MXan) and 4,9-dimethyl-8-hydroxylpsoralen (DMXan) were synthesized in order to clarify the effect of methyl on the antioxidant effectiveness of Xanthotoxol (8-hydroxylpsoralen, Xan), which were assessed by bleaching beta-carotene in linoleic acid-Triton emulsion, by interacting with 2,2'-azinobis(3-ethylbenzothiazoline-6-sulfonate) cationic radical (ABTS+), 2,2'-diphenyl-1-picrylhydrazyl radical (DPPH), and galvinoxyl radical, and by protecting DNA against the oxidation induced by Cu2+/glutathione (GSH) and 2,2'-azobis(2-amidinopropane hydrochloride) (AAPH). Methyl attaching to Xanthotoxol did not affect its ability to protect linoleic acid against autoxidation and to inhibit Cu2+/GSH-induced oxidation DNA, but decreased its ability to scavenge ABTS+ and DPPH, and to protect DNA against AAPH-induced oxidation. Therefore, methyl attenuated the antioxidant effectiveness of Xanthotoxol in radical-induced oxidation of DNA.
Xanthotoxol exerts neuroprotective effects via suppression of the inflammatory response in a rat model of focal cerebral ischemia.[Pubmed:23619720]
Cell Mol Neurobiol. 2013 Jul;33(5):715-22.
We previously found that Xanthotoxol, one of the major active ingredients in Cnidium monnieri (L.) Cusson, exerts protective effects in a rat model of focal cerebral ischemia/reperfusion injury by alleviating brain edema, inhibiting the neutrophil infiltration, and decreasing the expression of intercellular adhesion molecule-1 (ICAM-1) and E-selectin. The present study was designed to further determine the possible mechanisms of action of neuroprotective properties of Xanthotoxol after cerebral ischemia. Transient focal cerebral ischemia/reperfusion model in male Sprague-Dawley rats was induced by 2-h middle cerebral artery occlusion followed by 24-h reperfusion. Xanthotoxol (5 and 10 mg/kg) or vehicle were administered intraperitoneally at 1 and 12 h after the onset of ischemia. At 24 h after reperfusion, we assessed the effect of Xanthotoxol on the blood-brain barrier (BBB) permeability, the production of pro-inflammatory mediators such as interleukin (IL)-1beta, tumor necrosis factor (TNF)-alpha, IL-8, nitric oxide (NO), inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), and the p65 subunit of the transcription factor, nuclear factor-kappaB (NF-kappaB) in the cortex after ischemic insult. The results showed that Xanthotoxol treatment significantly attenuated BBB disruption, reduced the IL-1beta, TNF-alpha, IL-8 and NO level, and attenuated the iNOS activity compared with vehicle-treated animals. Further, Xanthotoxol treatment also significantly prevented the ischemia/reperfusion-induced increase in the protein expression of iNOS, COX-2, and the nuclear NF-kappaB p65. These results, taken together with those of our previous study, suggest that the neuroprotection may be attributed to the ability of Xanthotoxol to attenuate the expression of pro-inflammatory mediators and thereby inhibit the inflammatory response after cerebral ischemia.
Cytotoxic effect of xanthotoxol (8-hydroxypsoralen) on TCTC cells in vitro.[Pubmed:1437852]
Pol J Pharmacol Pharm. 1992 Jan-Feb;44(1):51-7.
The effect of Xanthotoxol (8-hydroxypsoralen) on proliferation of TCTC cells in vitro has been studied. Xanthotoxol at concentrations of 5 to 50 micrograms/ml inhibited the growth of cells. In cultures with Xanthotoxol, decreased amount of cell protein, mitotic index, and decreased ability to form a colony, were observed. Moreover, Xanthotoxol disturbed mitoses elevating the number of mitotic cells in the telophase stage. An increase of giant and multinuclear cells was also found. On the basis of these results it can be concluded, that 8-hydroxypsoralen which in comparison with other psoralens is not sensitive to photostimulation, inhibits the cell proliferation anyway. This fact shows that the mechanism of the psoralens activity is to some extent independent from the photostimulation.
[Calcium antagonistic effect of Xanthotoxol on isolated guinea pig atria].[Pubmed:16104510]
Zhong Yao Cai. 2005 Apr;28(4):319-21.
OBJECTIVE: To study the mechanism of depressant effect of Xanthotoxol (XT) on contractility in the isolated guinea pig atria. METHODS: The contractile force of the isolated left atria was determined by tension recording method. RESULTS: In the experiments on contractility of the left atria XT and Verapamil (Ver) significantly depressed the positive staircase phenomena, which was reversed by Ver but not by XT. However, the post-rest potentiation of myocardial contraction in the left atria was only markedly decreased by XT but not by Ver. Furthermore, XT not only attenuated the positive inotropic action, but also delayed the following toxicity response induced by ouabain in the isolated left atria. CONCLUSION: XT blocked not only the voltage dependent calcium channel, but also the receptor operated calcium channel in the isolated guinea pig atria.


